In vitro Assessment of the Biofield Treated Test Item on Cardiac Function Using Rat Cardiomyocytes Cell Line (H9c2) via Multiparametric Analysis
Abstract
Introduction
Heart disorders are the major concern of population health worldwide. According to WHO estimates 2018, 17.9 million peoples were died due to cardiovascular disorders.
Aim
The aim of this study was to investigate the cardioprotective activity of Biofield Energy Treated test item, Dulbecco's Modified Eagle Medium (DMEM) using rat cardiomyocytes (H9c2).
Methods
The test item (DMEM) was divided into three parts, first part received one-time Biofield Energy Treatment by a renowned Biofield Energy Healer, Mahendra Kumar Trivedi and was labeled as the one-time Biofield Energy Treated (BT-I) DMEM, while second part received the two-times Biofield Energy Treatment and is denoted as BT-II DMEM. The third part did not receive any treatment and defined as the untreated DMEM group.
Results
Cell viability of the test samples by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay showed 89.03% and 98.49% in the BT-I and BT-II groups, respectively suggested a nontoxic and safe in nature of the tested test item. The BT-I group showed 16.01% restoration of cell viability. The level of lactate dehydrogenase (LDH) was significantly inhibited by 50.37% and 49.35% in the BT-I and BT-II groups, respectively compared to the untreated DMEM group. Moreover, percent protection of creatine kinase-myocardial band (CK-MB) by 49.48% and 59.79% in the BT-I and BT-II groups, respectively, compared to the untreated DMEM group. Reactive oxygen species (ROS) level in terms of mean fluorescence unit (FU) was reduced by 6.64% in the BT-I group than untreated DMEM. Besides, BT-I and BT-II groups significantly increased the level of % apoptotic cells by 63.16% and 97.37% (p≤0.05), respectively than untreated DMEM.
Conclusion
Allover, results envisaged that Biofield Treatment significantly improved different cardiac parameters. Thus, Biofield Energy Treatment (The Trivedi Effect®) could be utilized as a cardio-protectant against several cardiac disorders such as coronary artery disease, heart attack, arrhythmias, heart failure, congenital heart disease, cardiomyopathy, etc.
Author Contributions
Academic Editor: Osmar Centurion, Professor of Medicine. Asuncion National University. Cardiology Division. First Department of Internal Medicine. Asuncion, Paraguay.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Mahendra Kumar Trivedi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Heart disorders are the major concern of population health worldwide. About 6 lakh peoples die due to heart disease in the United States every year; that’s 1/4 deaths 1. Cardiovascular disease (CVD) and stroke produce an immense health and economic burdens in the United States and globally. According to WHO estimates, in 2016, 17.9 million people around the globe die of cardiovascular diseases each year. This represents about 1/3 of all deaths globally 2, 3. CVD is the leading cause of death in Europe, accounting for over 4 million deaths each year. It has been projected that by 2020, CVD would be more numerous in India and China than in all economically developed countries in the world 4. Three main criteria to keep a healthy heart like opening blood vessels, strengthening the heart muscle, and controlling free radical damage by antioxidants 5. Apart from animal models and primary cardiac myocytes derived animal, even recent work has been done to develop human cardiomyocyte model systems for the screening of cardioprotective activity of substances 6. The use of in vitro test model for the prediction of heart damages provides several advantages over in vivo assessment. As this model require few animals, test material, and give high accuracy data 7. Rat cardiomyocytes cell line (H9c2) have been widely used as an alternative model to human cardiomyocytes in vitro for the assessment of cardio-protectant properties of any test substances 8.
Various study data suggested the effect of Energy Therapy in cancer patients through therapeutic touch 9, massage therapy 10, etc. Complementary and Alternative Medicine (CAM) therapies are preferred model of treatment, among which Biofield Therapy (or Healing Modalities) is one approach to enhance emotional, mental, physical, and human wellness. The National Center of Complementary and Integrative Health (NCCIH) has recognized and allowed Biofield Energy Healing as a CAM approach in addition to other therapies and medicines such as natural products, chiropractic/osteopathic manipulation, Qi Gong, deep breathing, Tai Chi, yoga, meditation, massage, special diets, healing touch, relaxation techniques, traditional Chinese herbs and medicines, naturopathy, movement therapy, homeopathy, progressive relaxation, guided imagery, pilates, acupuncture, acupressure, Reiki, rolfing structural integration, hypnotherapy, Ayurvedic medicine, mindfulness, essential oils, aromatherapy, and cranial sacral therapy. Human Biofield Energy has subtle energy that has the capacity to work in an effective manner 11. CAM therapies have been practiced worldwide with reported clinical benefits in different health disease profiles 12. This energy can be harnessed and transmitted by the experts into living and non-living things via the process of Biofield Energy Healing. Biofield Energy Treatment has been reported with a significant revolution in the field of cancer research 13, 14, materials science 15, 16, 17, microbiology 18, 19, 20, agriculture 21, 22, nutraceuticals 23, 24, and biotechnology 25, 26. Besides, The Trivedi Effect® also significantly improved bioavailability of various low bioavailable compounds 27, 28, 29, an improved overall skin health 30, 31, bone health 32, 33, 34, human health and wellness. Based on the excellent contribution of Biofield Energy in wide spectrum of areas, authors intend to extend the treatment modality to study the impact of the Biofield Energy Healing Treatment (The Trivedi Effect®) on the test item (DMEM) for cardiomyocytes cell line (H9c2).
Materials and Methods
Chemicals and Reagents
N-acetyl cysteine (NAC), 2′,7′-Dichlorofluorescin diacetate (DCFDA), 3-(4,5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT), and ethylenediaminetetraacetic acid (EDTA) were obtained from Sigma Chemical Co. (St. Louis, MO). Antibiotics solution (penicillin-streptomycin) was purchased from HiMedia, India. Dulbecco's Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco, India. Creatine kinase-MB (CK-MB) and lactate dehydrogenase (LDH) kits were obtained from Biovision, USA. Annexin-V kit was purchase from Guava Technologies, USA. The positive control, trimetazidine (TMZ) was procured from Zliesher Nobel, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Biofield Energy Healing Strategy
The test item (DMEM) was used in this experiment and one portion was considered as the untreated group, where no Biofield Treatment was provided. Further, the untreated group was treated with “sham” healer for comparison purpose. The sham healer did not have any knowledge about the Biofield Energy Healing Treatment. The other portion of the test item was received one-time Biofield Energy Treatment and referred as the BT-I and was also given two-times Biofield Energy Treatment and defined as the BT-II. Both the test items (BT-I and BT-II) were received Biofield Energy Healing Treatment (known as The Trivedi Effect®) under laboratory conditions for ~3 minutes through Mahendra Kumar Trivedi’s unique Biofield Energy Transmission process. Biofield Energy Healer was located in the USA, however the test items were located in the research laboratory of Dabur Research Foundation, New Delhi, India. Biofield Energy Healer in this experiment did not visit the laboratory, nor had any contact with the test samples. After that, the Biofield Energy Treated and untreated test items were kept in similar sealed conditions and used for the study as per the study plan.
Assessment of Cell Viability Using MTT Assay
The cell viability was performed by MTT assay in H9c2 cells (ATCC® CRL-1446™). The cells were counted and plated in a 96-well plate at the density corresponding to 10 X 103 cells/well/180 µL in DMEM + 10% FBS. The cells in the above plate(s) were incubated for 24 hours in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. Following incubation, the medium was removed and the following treatments were given. In the test item group, 200 µL of the test item was added to wells. Besides, in the positive control group, added 180 µL of SFM with 20 µL of positive controls were added from the respective 10X stock solutions. After incubation for 48 hours, the effect of test item on cell viability was assessed by MTT assay. 20 µL of 5 mg/mL of MTT was added to all the wells and incubated at 37°C for 3 hours. The supernatant was aspirated and 150 µL of DMSO was added to all wells to dissolve formazan crystals. The optical density (OD) of each well was read at 540 nm using Biotek Reader.
Effect of the test items on viability of H9c2 cells was determined using Equation (1):
% Cell viability =(100-% Cytotoxicity)……………..(1)
Where, % Cytotoxicity = {(O.D. of untreated cells – O.D. of cells treated with test item)/ OD of untreated cells}*100
The concentrations resulting in ≥70% cell viability were taken as safe/non-cytotoxic for cytokine estimation.
Evaluation of Cytoprotective Effect of the Test Item
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted on an hemocytometer and seeded at a density of 5 X 103 cells/well/180 µL in DMEM + 10% FBS in a 96-well plate. Cells were incubated in a CO2 incubator for 24 hours at 37°C, 5% CO2,and 95% humidity. After 24 hours, the medium was removed and the following treatments were given. In the test item group, 180 µL of the test item was added to wells. In the positive control group, 160 µL of SFM and 20 µL of positive control from the respective 10X stock solution was added to wells. After 24 hours of treatment, cells were treated with t-BHP at a final concentration of 250 μM (20 µL from the respective 10X stock) for 4 hours. After 4 hours, the protective effect of the test item on cell viability was assessed by MTT assay. The protective effect of the test item on survival of H9c2 cells against t-BHP induced damage was determined using Equation (2)
((A-B)/(C-B))*100……………(2)
Where, A = O.D. of test item/positive control + t-BHP treated cells
B= O.D. of cells (t-BHP alone)
C = O.D. of untreated cells
Estimation of Lactate Dehydrogenase (LDH)
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted (using hemocytometer) and seeded (at a density of 0.12 X 106 cells/well/500 µL) in DMEM + 10 % FBS in 48-well plates. Cells were incubated in a CO2 incubator for 24 hours at 37°C, 5 % CO2 and 95% humidity. After 24 hours, medium was removed and following treatments were given. Test items (BT-I and BT-II) groups (450 µL of Biofield Treated DMEM), positive controls (trimetazidine and N-acetyl cysteine) groups (400 µL of SFM), and (untreated DMEM) group (500 µL of SFM) were added to the respective wells and incubate for 24 hours. After that, cells were treated with 300 µM of t-BHP (50 µL from the respective 10X stock) for 2.5 hours. Supernatants were collected from each well and stored at -20°C till analyzed. Estimation of LDH in culture supernatants was done using Lactate Dehydrogenase Activity Colorimetric Assay Kit as per manufacturer’s instructions. LDH activity (nMoles/min/mL) was determined and the protective effect of test item was calculated using Equation (3):
((A-B)/(A-C))*100……………(3)
Where, A = LDH activity in cells (t-BHP alone)
B= LDH activity in test items/positive controls + t-BHP induced cells
C = LDH activity in untreated cells
Estimation of CK-MB
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted on a hemocytometer. Cells were seeded at a density of 0.12 X 106 cells/well/500 µL in DMEM + 10 % FBS in 48-well plates. Cells were incubated in a CO2 incubator for 24 hours at 37°C, 5 % CO2 and 95 % humidity. After 24 hours, medium was removed and treatments were given.
Test items (BT-I and BT-II) groups (450 µL of Biofield Treated DMEM), positive control (N-acetyl cysteine) group (400 µL of SFM), t-BHP per se group (450 µL of SFM), and negative control (untreated) group (500 µL of SFM) were added to the respective wells and incubate for 24 hours. After incubation for 24 hours, cells were treated with 300 µM of t-BHP (50 µL from the respective 10X stock) for 2.5 hours. Supernatants were collected from each well and stored at -20°C till analyzed. Estimation of CK-MB in culture supernatants was done using Creatine Kinase Activity Colorimetric Assay Kit as per manufacturer’s instructions. CK-MB activity (nMoles/min/mL) was determined and protective effect of test item on CK-MB activity was calculated using Equation (4):
((A-B)/(A-C))*100……………(4)
Where, A = CK-MB activity in cells (t-BHP alone)
B= CK-MB activity in test items/positive controls + t-BHP treated cells
C = CK-MB activity in untreated cells
Assessment of Reactive Oxygen Species (ROS)
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Then, the cells were counted with the help of a hemocytometer and seeded (at a density of 20 X 103 cells/well/180 µL in DMEM + 10 % FBS) in 96-well plates. Cells were incubated in a CO2 incubator for 24 hours at 37 °C, 5 % CO2 and 95 % humidity. Then, medium was removed and treatments were given. About 180 µL of the test item (TI), 160 µL of SFM, 180 µL of SFM, and 200 µL of SFM was added to wells of test items, positive controls, t-BHP per se, and untreated DMEM groups, respectively and incubate for 24 hours. After incubation for 24 hours, cells were stained with DCFDA and washed the wells once with Hank's Balanced Salt Solution (HBSS) + 2% FBS solution and 180 µL of SFM was added to each well. Protective effect of TI on ROS activity was calculated using Equation (5):
((A-B)/(A-C))*100……………(5)
Where, A = Mean FU in Control cells (t-BHP alone)
B= Mean FU in TI/positive control + t-BHP treated cells
C = Mean FU in untreated cells
Effect of Test Item on Apoptosis
Cells were trypsinized and a single cell suspension of H9c2 was prepared. Cells were counted using hemocytometer and seeded at a density of 0.25 million/well/1 mL in DMEM + 10% FBS in 96-well plates. Further, the cells were incubated in a CO2 incubator for 24 hours at 37°C, 5 % CO2 and 95% humidity. After 24 hours, medium was removed and the following treatments were given. The TI group received 900 µL of Tis (BT-I and BT-II), positive control (N-acetyl cysteine) group received 800 µL of SFM, t-BHP group received 900 µL of SFM, and the untreated DMEM group provided 1 mL of SFM to the corresponding wells and incubate for 24 hours. After that, cells were treated with 300 µM of t-BHP (100 µL from the respective 10X stock) for 2.5 hours. Then, the cells were stained with Annexin reagent for apoptotic population as follows: cells were gently harvested by trypsinisation into prelabeled centrifuge tubes followed by pelleted and resuspended in 200 µL of SFM. At 100 µL of cell suspension was stained with 100 µL of Annexin reagent for 30 minutes in a dark condition at room temperature. Cells were acquired at flow cytometer (Guava technologies). The protective effect of the TI was calculated using Equation (6):
((A-B)/(A-C))*100……………(6)
Where, A = % Apoptotic population in t-BHP
B = % Apoptotic population in test items/positive control + t-BHP treated cells
C = % Apoptotic population in untreated cells
Statistical Analysis
All the values were represented as Mean ± SEM (standard error of mean) of three independent experiments. The statistical analysis was performed using SigmaPlot statistical software (v11.0). For two groups comparison student’s t-test was used. For multiple group comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p≤0.05.
Results and Discussion
Cell Vability Using MTT Assay
Evaluation of cell viability after treatment with the positive controls and the test items in H9c2 cells is shown in Figure 1 The cardioprotective activity of Biofield already been published by Branton A, 2019 35. The positive controls, trimetazidine (TMZ) showed more than 88% at the concentrations between 0.1 to 100 µg/mL and N-acetyl cysteine (NAC) showed greater than 74% cell viability upto 500 µg/mL. Besides, the Biofield Energy Treated test items, BT-I (one-time Biofield Energy Treated DMEM) and BT-II (two-times Biofield Energy Treated DMEM) showed 89.03% and 98.49% cell viability, respectively. Overall, the Biofield Energy Treated test items found as a safe and non-toxic profile of the test substances and further used in this experiment for the assessment of various cardiac parameters.
Figure 1.Effect of the test items and positive controls on cell viability in H9c2 cells after 24 hours of treatment. UNT: Untreated; TMZ: Trimetazidine; NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
Evaluation of Cytoprotective Effect of the Test Item
For the assessment of cardioprotective activity of the test compounds, tert-butyl hydroperoxide (t-BHP) is a well-recognized oxidative stress inducer in the in vitro cell-based assays 36, 37. The cytoprotective activity of the Biofield Energy Treated test items on the restoration of cell viability in H9c2 cells was determined against t-BHP induced cell damage and the result is shown in Figure 2. Trimetazidine (TMZ) resulted, restoration of cell viability by 54.1%, 41.3%, 14.33%, and 4.16% at 0.1, 1, 10, and 50 µg/mL, respectively compared to the t-BHP induced group. Besides, the test group’s like the one-time Biofield Energy Treated DMEM (BT-I) showed 16.01% and two-times Biofield Energy Treated DMEM exhibited 4.43% restoration of cell viability with respect to the t-BHP induced group. The cellular antioxidant capacity can reduced due to excess production of free radicles that leads to inflammation 38. This excess levels of free radicles can affect the normal functions of cell membrane, and ultimately altered the genetic materials and cause various age-related disorders such as diabetes, cardiovascular, autoimmune diseases, and cancer 39, 40, 41. The results suggest that Biofield Treatment has significantly protects t-BHP induced cardiotoxicity, which could be due to The Trivedi Effect®. Therefore, Biofield Energy Healing Treatment could be used for the management of cardiovascular disorders.
Figure 2.Assessment of cytoprotective effect of the test items in H9c2 cells against tert-butyl hydroperoxide (t-BHP) induced damage. TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM
Estimation of Lactate Dehydrogenase (LDH)
The distribution of lactate dehydrogenase (LDH) is mainly abundant in the heart and skeletal muscle, is a tetrameric enzyme, and is mainly responsible for anaerobic respiration of cells 42, 43, 44. The effect of test items on the level of lactate dehydrogenase (LDH) is presented in Figure 3. The level of LDH activity was significantly increased by 892.05% in the tert-butyl hydroperoxide (t-BHP) induced group as compared to the untreated DMEM group (3.65 ± 0.5 nMoL/min/mL). The positive control, trimetazidine (TMZ) exhibited 8.04%, 18.64%, and 96.13% inhibition of lactate dehydrogenase (LDH) compared to the untreated DMEM group. Besides, One-time Biofield Energy Treated DMEM group (BT-I) and two-times Biofield Energy Treated DMEM group (BT-II) group showed 50.37% and 49.35%, respectively as compared to the untreated DMEM group.
Figure 3.The effect of the test items on lactate dehydrogenase (LDH) against tert-butyl hydroperoxide (t-BHP) induced damage. TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM.
Estimation of Creatine Kinase-Myocardial Band (CK-MB)
The impact of the Biofield Energy Treated test items on cardiac marker, creatine kinase-myocardial band (CK-MB) is shown in Figure 4. The level of CK-MB was significantly increased by 4.96% in the tert-butyl hydroperoxide (t-BHP) induced group as compared to the untreated DMEM group (1.14 ± 0.16 nMol/min/mL). The positive control, N-acetyl cysteine (NAC) showed 4.86%, 24% (p≤0.001), and 90.57% (p≤0.001) significant inhibition of CK-MB enzyme activity in a concentration-dependent manner at 25, 50, and 100 µM, respectively compared to t-BHP induced group. Further, the Biofield Treated test items group, BT-I (one-time Biofield Energy Treated DMEM) and BT-II (two-times Biofield Energy Treated DMEM) showed significant (p≤0.001) inhibition of CK-MB enzyme level by 49.48% and 59.79%, respectively as compared to the t-BHP induced group. This reduction of tissue-specific cardiac biomarker (CK-MB) is very essential for the diagnosis of cardiac functions apart from of troponin T (cTnI) and myoglobin (Myo) 45, 46. As CK-MB is a sensitive and specific indicator for the diagnosis of an acute myocardial infarction (AMI) 47. Overall, the Biofield Treated test items (BT-I and BT-II) has significantly inhibited the levels of cardiac tissue-specific enzyme CKMB, which was induced by t-BHP.
Figure 4.The effect of the test items (24 hours of pretreatment) on Creatine Kinase-Myocardial Band (CK-MB) activity against tert-butyl hydroperoxide (t-BHP) induced damage after 4 hours of treatment. NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM. ***p≤0.001 vs. t-BHP at 300 µM.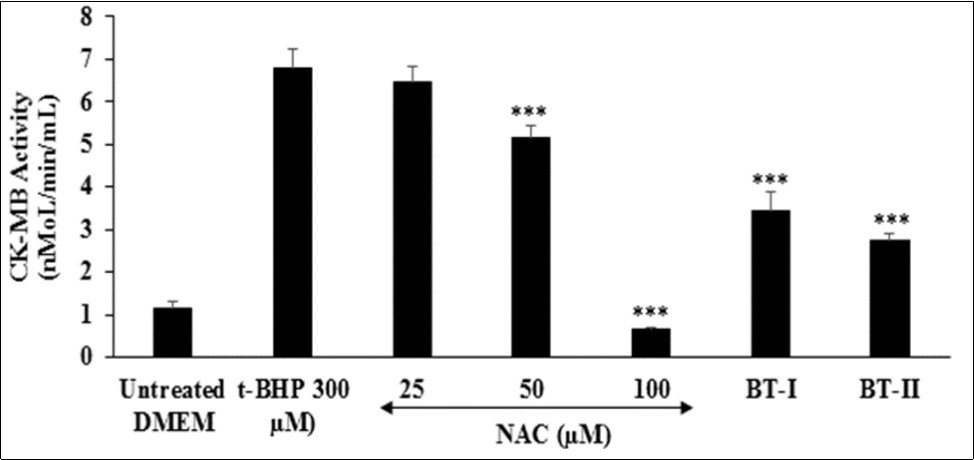
Assessment of Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) play a vital role in the development of cardiovascular disorders specifically atherosclerosis 48. Due to misbalance of ROS production and antioxidant defense capacity results in oxidative stress 49, 50. Although ROS is normally required in cell signaling pathways, while excessive production of ROS leads to cell damage 51. The number of fluorogenic substrates that serve as hydrogen donors have been used in conjunction with horseradish peroxidase (HRP) enzyme to produce intensely fluorescent products 52. In this assay, the effect of the Biofield Treated test items on the ROS induced by t-BHP in terms of number of fluorescence unit (FU) is shown in Figure 5. The positive control, trimetazidine (TMZ) showed 5.46% reduction of FU at 25 µM as compared to the untreated DMEM group. Further, one-time Biofield Energy Treated DMEM (BT-I) showed 6.64% reduction of mean FU compared to the untreated group. Results found that the BT-I have significantly protect cardiomyocytes from oxidative stress. This inhibition of ROS could be due to the Biofield Energy Treatment through the change in protons and neutrons in the nucleus caused by weak interactions.
Figure 5.The effect of the test items on reactive oxygen species in terms of fluorescence unit (FU) in H9c2 cells after 24 hours of treatment. FU: Fluorescence unit; TMZ: Trimetazidine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM.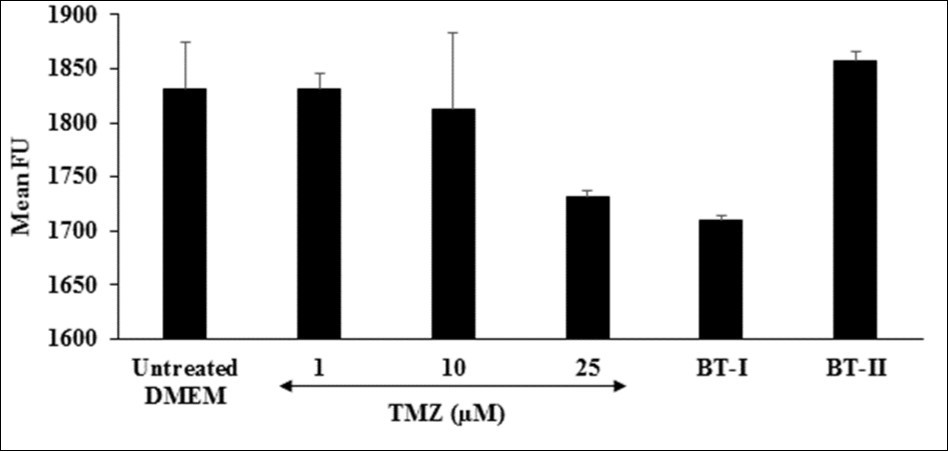
Effect of Test Item on Apoptosis
Cardiovascular disorders is the leading cause of morbidity and mortality in the developed world. Apoptosis, a process of programme cell death that plays a vital role in various pathologic conditions related to cardiovascular system 53. Inhibition of apoptotic pathway is one of the potential treatment approach for the management of cardiovascular disorders 54, 55. The apoptotic process ensures that damaged, aged, or excess cells are deleted in a regulated manner that is not harmful to the host 56. Thus, increased level of percent apoptotic cells is directly link with the overall health. The effect of the test items on the level of percent apoptotic cells is shown in Figure 1. The positive control, N-acetyl cysteine (NAC) was significantly increased the level of percent apoptotic cells by 94.08%, 77.63%, and 57.89% at 100, 200, and 300 µM, respectively compared to the untreated DMEM group. Further, Biofield Treated test items BT-I (one-time Biofield Energy Treated DMEM) and BT-II (two-times Biofield Energy Treated DMEM) showed significantly increased the percent of apoptotic cells by 63.16% and 97.37% (p≤0.05), respectively compared to untreated DMEM. Overall, results suggested that Biofield Energy can increase the level of percent apoptotic cells, which could be able to remove damaged, unwanted, aged, and excess cells from the body Figure 6.
Figure 6.Effect of the test items on percent apoptotic cells in H9c2 cells after 24 hours of treatment. NAC: N-acetyl cysteine; BT-I: One-time Biofield Energy Treated DMEM; BT-II: Two-times Biofield Energy Treated DMEM. *p≤0.05 vs. untreated DMEM.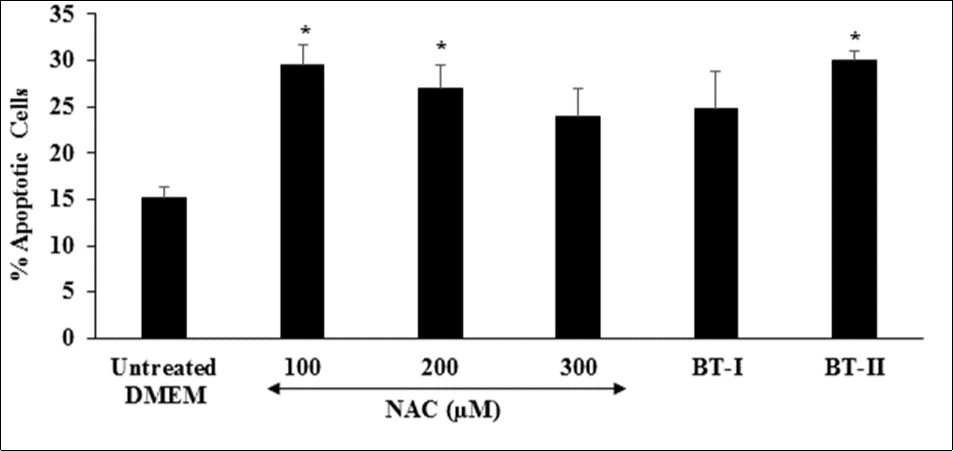
Conclusions
The study outcomes showed that the test substances were safe and non-toxic based on MTT cell viability assay with more than 89% viable cells. The one-time Biofield Energy Treated DMEM (BT-I) showed 16.01% cytoprotective activity. Further, increased level of lactate dehydrogenase (LDH) was significantly suppressed by 50.37% in the BT-I group, and 49.35% in the BT-II (two-times Biofield Energy Treated DMEM) group as compared to the untreated DMEM group. The cardio-specific enzyme, creatine kinase-myocardial band (CK-MB) was significantly inhibited by 49.48% and 59.79% in the BT-I and BT-II, respectively compared to the untreated DMEM group. Moreover, Reactive oxygen species (ROS) level in terms of mean fluorescence unit (FU) was reduced by 6.64% in the BT-I group than untreated DMEM. Percent apoptotic cells were significantly increased by 63.16% and 97.37% in the BT-I and BT-II, respectively compared to the untreated DMEM. In conclusion, The Biofield Energy Treatment significantly improved various cardiac parameters and protect cardiomyocytes cells from oxidative damage. Thus, it can be used as a complementary and alternative treatment for the prevention of various types of cardiac disorders viz. High blood pressure (hypertension), stroke, congestive heart failure (CHF), peripheral artery disease, congenital heart disease, rheumatic heart disease, valvular heart disease, carditis, thromboembolic disease, and venous thrombosis, etc. Further, it could be useful to improve cell-to-cell messaging, normal cell growth and differentiation, cell cycling and proliferation, neurotransmission, skin health, hormonal balance, immune and cardiovascular functions. Moreover, it can also be utilized in organ transplants (i.e., kidney, liver, and heart transplants), hormonal imbalance, aging, and various inflammatory and immune-related disease conditions like Alzheimer’s Disease (AD), Ulcerative Colitis (UC), Dermatitis, Asthma, Irritable Bowel Syndrome (IBS), Hashimoto Thyroiditis, Pernicious Anemia, Sjogren Syndrome, Multiple Sclerosis, Aplastic Anemia, Hepatitis, Graves’ Disease, Dermatomyositis, Diabetes, Parkinson’s Disease, Myasthenia Gravis, Atherosclerosis, Systemic Lupus Erythematosus (SLE), stress, etc. with a safe therapeutic index to improve overall health and Quality of Life.
Acknowledgements
Authors gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, Trivedi testimonials and Trivedi master wellness for their support. In addition, authors are thankful for the support of Dabur Research Foundation for conducting this study.
References
- 1.CDC NCHS.Underlying Cause of Death 1999-2013 on CDC WONDER Online Database, released 2015. Data are from the Multiple Cause of Death Files, 1999-2013, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program.AccessedFeb.3,2015.
- 3.Benjamin E J, Virani S S, Callaway C W, Chang A R, Cheng S. (2018) Heart disease and stroke statistics-2018update:A report from the American Heart Association Circulation.DOI-10.1161/CIR.0000000000000558.
- 5.Rakesh S, Arunporn I. (2017) Herbal supplements or herbs in heart disease: Herbiceutical formulation, clinical trials, futuristic developments. , J Cardiol Cardiovasc 3(1), 555603.
- 6.Peter A K, Bjerke M A, Leinwand L A. (2016) . Biology of the cardiac myocyte in heart disease. Drubin DG, ed. Molecular Biology of the Cell.27(14),2149-2160 .
- 7.Kuznetsov A V, Javadov S, Sickinger S, Frotschnig S, Grimm M. (2015) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. , Biochimica et Biophysica Acta.1853(2),276-284
- 8.Duthie S J, Melvin W T, Burke M D. (1994) Bromobenzene detoxification in the human liver-derived HepG2 cell line. , Xenobiotica 24, 265-279.
- 9.Lutgendorf S K, Mullen-Houser E, Russell D, Degeest K, Jacobson G. (2010) Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun.24,1231-1240.
- 10.Ironson G, Field T, Scafidi F, Hashimoto M, Kumar M. (1996) Massage therapy is associated with enhancement of the immune system's cytotoxic capacity. , Int J Neurosci 84, 205-217.
- 11.Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med.4,58-66.
- 12.Rubik B. (2002) The biofield hypothesis: Its biophysical basis and role in medicine. , J Altern ComplementMed8 703-717.
- 13.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. , J Integr Oncol.4,141
- 14.Trivedi M K, Patil S, Shettigar H, Gangwar M, Jana S. (2015) In vitro evaluation of biofield treatment on cancer biomarkers involved in endometrial and prostate cancer cell lines. , J Cancer Sci Ther 7, 253-257.
- 15.Trivedi M K, Tallapragada R M. (2008) A transcendental to changing metal powder characteristics. Met Powder Rep.63,22-28,31.
- 16.Trivedi M K, Nayak G, Patil S, Tallapragada R M, Latiyal O. (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage.4,161.
- 17.Trivedi M K, Nayak G, Patil S, Tallapragada R M, Latiyal O. (2015) Effect of biofield energy treatment on physical and structural properties of calcium carbide and praseodymium oxide. , International Journal of Materials Science and Applications.4,390-395
- 18.Trivedi M K, Branton A, Trivedi D, Nayak G, Charan S. (2015) Phenotyping and 16S rDNA analysis after biofield treatment onCitrobacterbraakii: A urinary pathogen. , J Clin Med Genom.3,129
- 19.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. , J Antivir Antiretrovir.7,83-88
- 20.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) An impact of biofield treatment: Antimycobacterial susceptibility potential using BACTEC 460/MGIT-TB System. Mycobact Dis.5,189.
- 21.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (MangiferaindicaL.). , Journal of Food and Nutrition Sciences 3, 245-250.
- 22.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C. (2015) Evaluation of biochemical marker – Glutathione and DNA fingerprinting of biofield energy treatedOryza sativa. , American Journal of BioScience.3,243-248
- 23.Trivedi M K, Branton A, Trivedi D, Nayak G, Plikerd W D. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. , International Journal of Bioorganic Chemistry.2,135-145
- 24.Parulkar V R, Trivedi M K, Branton A, Trivedi D, Nayak G. (2018) Improved metabolism of vitamin d3 in human osteoblasts cells after biofield energy healing treatment. , American Journal of Laboratory Medicine.3,11-19
- 25.Trivedi M K, Patil S, Shettigar H, Bairwa K, Jana S. (2015) Phenotypic and biotypic characterization ofKlebsiellaoxytoca: An impact of biofield treatment. , J Microb Biochem Technol 7, 203-206.
- 26.Nayak G, Altekar N. (2015) Effect of biofield treatment on plant growth and adaptation. , J Environ Health Sci.1,1-9
- 27.Branton A, Jana S. (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. , International Journal of Clinical and Developmental Anatomy 3, 9-15.
- 28.Branton A, Jana S. (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. , American Journal of Clinical and Experimental Medicine 5, 138-144.
- 29.Branton A, Jana S. (2017) Effect of The biofield energy healing treatment on the pharmacokinetics of 25-hydroxyvitamin D3[25(OH)D3] in rats after a single oral dose of vitamin D3. , American Journal of Pharmacology and Phytotherapy 2, 11-18.
- 30.Parulkar V R, Trivedi M K, Branton A, Trivedi D, Nayak G. (2017) The use of consciousness energy healing based herbomineral formulation for skin anti-aging strategies. , Journal of Food and Nutrition Sciences 5, 96-106.
- 31.Singh J, Trivedi M K, Branton A, Trivedi D, Nayak G. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. , American Journal of Pharmacology and Phytotherapy 2, 1-10.
- 32.Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. , International Journal of Biomedical Engineering and Clinical Science 4, 6-14.
- 33.Lee A C, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) The potential benefits of biofield energy treated vitamin D3on bone mineralization in human bone osteosarcoma cells (MG-63). , International Journal of Nutrition and Food Sciences 7, 30-38.
- 34.Stutheit M E, Trivedi K, Branton A, Trivedi D, Nayak G. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. , American Journal of Life Sciences.6,13-21
- 35.Branton A, Jana S. (2019) Improved metabolic cardiac biomarkers activity using rat cardiomyocytes cell line (H9c2) against biofield energy treated test sample. , J Cardiol.3(1),000137
- 36.Alía M, Ramos S, Mateos R, Bravo L, Goya L. (2005) Response of the antioxidant defense system totert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J Biochem Mol Toxicol.19,119-128.
- 37.Vargas-Mendoza N, Madrigal-Santillán E, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C. (2014) Hepatoprotective effect of silymarin. , World J Hepatol 6, 144-149.
- 38.Webb C, Twedt D. (2008) Oxidative stress and liver disease. Vet Clin North Am Small Anim Pract.38,125-135.
- 39.Li Sha.Hor-Yue Tan, Ning Wang, Zhang-Jin Zhang, Lixing Lao et al. (2015) The role of oxidative stress and antioxidants in liver diseases. , Int J Mol Sci 16, 26087-26124.
- 40.Cheresh P, Kim S J, Tulasiram S, Kamp D W. (2013) Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta.1832,1028-1040.
- 41.Lu L Y, Ou N, Lu Q B. (2013) Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci Rep.3,3169.
- 42.Burgner J W, Ray W J. (1984) On the origin of the lactate dehydrogenase induced rate effect. , Biochemistry 23, 3636-3648.
- 43.Valvona C J, Fillmore H L, Nunn P B, Pilkington G J. (2015) The regulation and function of lactate dehydrogenase A: Therapeutic potential in brain tumor. , Brain Pathol 26, 3-17.
- 44.Kopperschläger G, Kirchberger J. (1996) Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. , J Chromatogr B Biomed 684(12), 25-49.
- 45.Arram E O, Fathy A.Abdelsamad AA, Elmasry EI (2014) Value of cardiac biomarkers in patients with acute pulmonary embolism. , Egypt J Chest Dis 63(1), 247-252.
- 46.Wang J, Wang F. (2017) The detection value of CK-MB, Myo and cTnIin in patients with AMI and HF. Biomed Res.28(19),8533-8536.
- 47.Guzy P M. (1977) Creatine phosphokinase-MB (CPK-MB) and the diagnosis of myocardial infarction. , West J 127(6), 455-460.
- 48.He F, Zuo L. (2015) Redox roles of reactive oxygen species in cardiovascular diseases. Miller FJ, ed , Int J Mol 16(11), 27770-27780.
- 49.Halliwell B. (1984) Oxygen radicals: A commonsense look at their nature and medical importance. , Med Biol.62(2),71-77
- 50.Zuo L, Best T M, Roberts W J, Diaz P T, Wagner P D. (2015) Characterization of reactive oxygen species in diaphragm. , Acta Physiol (Oxf) 213(3), 700-710.
- 51.Zuo L, Shiah A, Roberts W J, Chien M T, Wagner P D. (2013) Low Po conditions induce reactive oxygen species formation during contractions in single skeletal muscle fibers. , Am J Physiol Regul Integr Comp 304(11), 1009-1016.
- 52.Tarpley M M, Wink D A, Grisham M B. (2004) Methods for detection of reactive metabolites of oxygen and nitrogen:In vitroandin vivoconsiderations. , Am J Physiol Regul Integr Comp Physiol.286,R431-R444
- 54.Saraste A, Voipio-Pulkki L M, Parvinen M, Pulkki K. (1997) Apoptosis in the heart. , N Engl J Med.336 1025-1026.
Cited by (6)
- 1.Trivedi Mahendra Kumar, Branton Alice, Trivedi Dahryn, Jana Snehasis, 2021, Evaluation of Cardiac Performance after Treatment with the Biofield Energy Treated Proprietary Test Formulation on L-NAME and High Fat Diet-Induced Cardiovascular Disorders in Sprague Dawley Rats, Journal Of Hypertension And Cardiology, 3(2), 6, 10.14302/issn.2329-9487.jhc-21-3848
- 2.Trivedi Mahendra Kumar, Branton Alice, Trivedi Dahryn, Jana Snehasis, Ahmed Riaz, 2021, Evaluation of Inflammatory Serum Cytokines after Treatment with the Consciousness Energy Healing Based Proprietary Test Formulation on Combination of Cecal Slurry, LPS and E. Coli Induced Systemic Inflammatory Response Syndrome (SIRS) in Sprague Dawley Rats, Journal of Current Scientific Research, 1(3), 23, 10.14302/issn.2766-8681.jcsr-21-3885
- 3.Trivedi Mahendra Kumar, Branton Alice, Trivedi Dahryn, Jana Snehasis, BAJAJ ANUBHA, 2021, Antioxidant and Anti-Inflammatory Activities of Biofield Energy Treated Proprietary Test Formulation in Brain Tissues in Cecal Slurry, LPS and E. Coli-Induced Systemic Inflammatory Response Syndrome (SIRS) in Sprague Dawley Rats, International Journal of Global Health, 1(3), 13, 10.14302/issn.2693-1176.ijgh-21-3886
- 4.Trivedi Mahendra Kumar, Branton Alice, Trivedi Dahryn, Jana Snehasis, Stoleski Sasho, 2021, Evaluation of Renal and Cardioprotective Potential of the Biofield Energy Treated Proprietary Test Formulation on L-NAME and High Fat Diet-Induced Cardiovascular Disorders in Sprague Dawley Rats, Journal of Nephrology Advances, 1(3), 12, 10.14302/issn.2574-4488.jna-21-3847
- 5.Trivedi Mahendra Kumar, Branton Alice, Trivedi Dahryn, Jana Snehasis, 2021, Evaluation of Antioxidative Potential of the Biofield Energy Treated Proprietary Test Formulation on L-NAME and High Fat Diet-Induced Cardiovascular Disorders in Sprague Dawley Rats, Journal of Antioxidant Activity, 2(2), 1, 10.14302/issn.2471-2140.jaa-21-3846
- 6.Frolova Sofia, Gordienko Olena, Yarmolenko Olha, 2022, THE EFFECT OF ALLOXAN-INDUCED HYPERGLYCEMIA ON THE RENAL CORTEX, Eastern Ukrainian Medical Journal, 10(3), 268, 10.21272/eumj.2022;10(3):268-273
