Abstract
The effect of resveratrol, a free radical scavenger, during cataract development was evaluated in the Wistar rat pup model. This study investigated the possible free radical scavenging potential of resveratrol at 40 mg/ kg body wt dose in selenite-induced cataract in rat pups. Intraperitoneal injection of sodium selenite (15 µm mol/ kg body wt) in 8 to 10 day old rat pups lead to severe oxidative stress in the tissues evidenced by decreased antioxidants and increased lipid peroxidase, nitric oxide, superoxide anion, hydroxyl radical generation, inducible nitric oxide synthase (iNOS) as well as nuclear factor kappa B (NF-kB) expression levels that probably led to cataract formation. Selenite exposure also caused an increase in total calcium in the eye lens and significantly inhibited the activity of Ca2+ ATPase but not Na+/ K+ ATPase or Mg2+ ATPase. However, both pre- and co-treatments with resveratrol, but not post-treatment, led to an increase in antioxidant levels with a concomitant reduction in oxidative stress and also rescued the selenite-mediated increase in lens Ca2+ and inhibition of Ca2+ ATPase activity in the eye lens. The results of this study demonstrate antioxidants decrease and increase in free radical generation triggered by selenite causes the inactivation of lens Ca2+ ATPase leading to a rise in intracellular Ca2+ level. Resveratol treatment was able to prevent selenite-induced oxidative stress and in turn the inhibition of lens opacification. Thus, resveratrol has the potential to function as an anti-cataractogenic agent, possibly by preventing free radical-mediated accumulation of Ca2+ in the eye lens.
Author Contributions
Academic Editor: Asaad Ghanem, Mansoura ophthalmic center, mansoura university, mansouraam
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 R Manikandan
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Cataract is the most common cause of treatable blindness worldwide and develops as a result of progressive loss in lens transparency 1, 2, 3. It has been demonstrated that oxidative stress plays an important role in cataract etiopathogenesis, as with many age-related diseases 4, 5, 6, 7, 8, 9, 10. Oxidative stress occurs when free radical formation exceeds the cellular antioxidant capacity. Cells have evolved to combat these free radicals, utilizing different antioxidant enzymes 2. The connection of these early effects to subsequent opacification of the lens tissue that is characterized by the presence of disulfide liked proteins of higher molecular weight with lower solubility 11 is quite complex. Such crosslinks are primarily established between lens glutathione and proteins, especially the lens crystallins. In addition, membrane lipids are also peroxidized 12 causing cell damage. Cataract formation may be attributed to oxidative stress triggered by reactive oxygen species (ROS), which include, superoxide anion (O2-), nitric oxide (NO), hydrogen peroxide (H2O2), peroxynitrite (ONOO-) and hydroxyl radical (OH.). Some of these free radicals may react with each other or other cellular components to yield highly reactive compounds that cause extensive cellular damage 13.
Nitric oxide (NO) is an important signalling molecule that mediates a variety of essential physiological processes, including neurotransmission, vasodilatation and host cell defense. NO is synthesized from L-arginine by NO synthase (NOS). While an inducible nitric oxide synthase (iNOS), one of the three isoforms of NOS, is usually not present under normal conditions, and has been known to be induced by cytokines, advanced glycation end products in addition to the transcription factor, nuclear factor-kappa B (NF-κB) 14, 15 to name a prominent few. Because iNOS promoter has an NF-κB binding site, the activation of NF-κB is important for iNOS induction 16, 17, 18, 19, 20, 21. The induction of iNOS could result in elevated and sustained release of NO, which can be cytotoxic 22, 23. Of particular relevance is the finding that NO is known to influence apoptosis in a variety of animal models of disease 24. The over production of NO in response to induction of iNOS is observed in ureitis, vetinins, glaucoma 25, 26 and cataract 27, 28.
The calcium level in lens cells is essential for lens physiology, and maintenance of lens transparency. Calcium increase is associated with cataracts in humans and most animal models 29, 30. It has been shown that selenite cataractogenesis involves Ca2+mediatedactivation of calpain II, a Ca2+-dependent protease, and irreversible damage of the lens nuclease 31, but the underlying mechanism could very well involve the progressive deterioration in Ca2+ homeostasis 32 that leads to disease. We would like to highlight a few reports that have shown that calpain participates in the regulation of lens Ca2+ homeostasis 33, 34, 35, 36. Although the physiological function of calpain is not exactly clear, it has been suggested to play important roles in cellular functions that occur in response to mobilized calcium ions 37. Thus, it seems likely that calpain is involved not only in physiological events but also in various pathological states, including cataract development 38.Although cataract surgery is recognized as being safe, there is a significant rate of complications leading to irreversible blindness. Thus, much emphasis is being laid on identification of compounds that will help to prevent cataractogenesis in the first place. Natural and synthetic compounds have been shown to prevent cataract formation induced by selenite and other chemicals 39, 40, 41. In these studies aimed at the developmental stages of cataract, there is a strong scientific basis for therapy using antioxidant and anti-inflammatory properties of specific compounds. Resveratrol (3, 4, 5- trihydroxy-trans-stibene), is a naturally occurring phytoalexin, found in grapes and variety of medicinal plants, is a stilbene derivative with three hydroxyl groups. The richest source of this compound is Polygonum cuspidatuma plant that has been used in oriental folk medicine against supportive dermatitis, gonorrhoea, favus, athlete’s foot and hyperlipemia 42. It has many biological and pharmaceutical properties, as evidenced through different experimental studies. For instance, resveratrol has been reported for its anti-tumor effects, increased cyclic adenosine monophosphate (cAMP) formation 43 and the inhibition of protein kinase C 44. It inhibits lipid peroxidation, angiogenesis, scavenges free radicals and modulates lipid as well as lipoprotein metabolism 45, 46, 47, 48, 49. Further, resveratrol has also been observed to play a role in the prevention of cardiovascular disease 50, 51. In this study we have made an attempt to unravel the use of resveratrol as a potential anti-cataract agent by analysing its effect on pro-inflammatory changes in eye lens.
Materials and Methods
Chemicals
Resveratrol and sodium selenite were purchased from Sigma Chemicals (St. Louis, MO, USA). Polyclonal rabbit anti-iNOS and NF-κB p65 antibodies were purchased from BD Biosciences (San Jose, CA, USA). Primers for iNOS and the HRP conjugated IgG secondary antibodies were purchased from Bangalore Genei (Bangalore, India). All other chemicals and reagents used were of the highest analytical grade commercially available.
Animals
Male albino Wistar rat pups 8-10 days of age and weighing 15-20 g were procured from the National Institute of Nutrition (Hyderabad, India). All experiments were approved by the Institutional Animal Ethical Committee (IAEC; No. 360/01/a/CPCSEA), India, guidelines. Rat pups were housed in an air-conditioned room at 22 ± 10°C with a lighting schedule of 12 h light and 12 h dark. Rat pups were fed a balanced commercial rat diet (Hindustan UniLever, Mumbai, India) and water ad libitum.
A pilot study was performed to determine the LD50 value for sodium selenite in rat pups 52. We observed the induction of cataract in suckling rat pups administered a single dose of sodium selenite s.c. (15 μmol/kg body wt) at around 10-12 days post partum with lens opacity evident in both eyes and this procedure was adopted for subsequent studies.
The rat pups were divided into five groups (of six animals each): group I, control rat pups receiving physiological saline; group II, selenite treated (single dose administered sub-cutaneously at 15 μmol/kg body weight); group III, selenite-induced group co-treated with resveratrol (single dose of resveratrol gavaged orally at 40 mg/kg body weight); group IV, selenite-induced animals post-treated (after 24 h) with resveratrol at the same dose as for group III; group V, rat pups pre-treated with resveratrol (same dose as for group III), 24 h before the administration of selenite.
Tissue Preparation
After treatment, rat pups were sacrificed by means of an overdose of pentobarbital at a dose of 50 mg/kg body weight given intraperitoneally. The lens tissue was immediately harvested, washed in ice-cold saline to remove blood, and frozen at -70°C. This was then homogenized using 10% 0.1 M Tris-HCl buffer (pH 7.2) and centrifuged (12,000 rpm, 30 min, 4°C). The supernatant obtained was estimated by the method of Lowry et al. 53 and used for further analyses.
Quantitative Analysis of Enzyme Activities
Catalase (CAT)
CAT activity was determined by the method of Beers and Sizer 54. In this test, dichromatic acetic acid is reduced to chromic acetate when heated in the presence of H2O2, with the formation of perchloric acid as an unstable intermediate. In the test, green colour development was read at 590 nm against blank in a spectrophotometer. The activity of catalase was expressed as µmol of H2O2 consumed/mg protein/min.
Superoxide Dismutase (SOD)
SOD activity was determined by the method of Misra and Fridovich 55. In this test, the degree of inhibition of pyrogallol auto-oxidation by serum and lens homogenate supernatant was measured. The change in absorbance was read at 470 nm against blank every 3 min on a spectrophotometer and the enzyme activity was expressed as 50% inhibition of adrenaline auto oxidation/min.
Glutathione Peroxidase (GPx)
The GPx activity was determined essentially as described by Rotruck et al. 56. The rate of glutathione oxidation by H2O2, as catalysed by GPx present in the supernatant is determined and colour developed was read against a reagent blank at 412 nm using a spectrophotometer. In the test, the enzyme activity was expressed as µmole of glutathione oxidized/mg protein/min.
Glutathione-S-transferase (GST)
The GST activity was determined by the method of Habig et al. 57. The conjugation of GSH with 1-chloro-2, 4-dinitrobenzene (CDNB), a hydrophilic substrate was observed spectrophotometrically at 340 nm to measure the GST activity and result was expressed in conjugate/µmol of CDNB with GSH/min/ mg protein.
Reduced Glutathione (GSH)
The GSH content was estimated by the method of Moron et al. 58. The serum and lens homogenate was centrifuged at 5000 rpm for 15 min at 4°C. To the resulting supernatant, 0.5 ml of 10% trichloroacetic acid was added and centrifuged. The resulting protein-free supernatant was allowed to react with 4 ml of 0.3 M Na2HPO4 (pH 8.0) and 0.5 ml of 0.04% (w/v) 5.5-dithiobis-2-nitrobenzoic acid. The absorbance of the resulting yellow colour was read spectrophotometrically at 412 nm and results were expressed as µmol of NADPH oxidized/min/mg.
Estimation of Superoxide Anion Generation in Eye Lens
The generation of O2- in the eye lens of Wistar rat pups was estimated spectrophotometrically using the cytochrome c method 59. Briefly, clear eye lens obtained from the various experimental groups was incubated with 500 μl phosphate buffer (pH 7.8, 0.1 M EDTA) and 100 μl of cytochrome c (0.002 mM) with or without superoxide dismutase (SOD), for 15 min. At the end of the reaction the absorbance was read at 550 nm in a UV-160 A Shimadzu spectrophotometer against a suitable blank and O2- generated was expressed as absorbance at 550 nm/15 min.
Determination of the Lipid Peroxidation Product, Malondialdehyde (MDA)
Lipid peroxidation was determined by the method of Ohkawa et al. 60. The principle of this method being that malondialdehyde (MDA), an end product of lipid peroxidation, reacts with thiobarbituric acid (TBA) to form a pink chromogen. For this assay, 0.2 ml of 8.1% SDS, 1.5 ml of 20% acetic acid (pH 3.5) and 1.5 ml of 0.8% thiobarbituric acid aqueous solution were added in succession in a reaction tube. To this reaction mixture, 0.2 ml of the serum and lens homogenate was added, and the mixture was then heated in boiling water for 60 min. After cooling to room temperature, 5 ml of butanol: pyridine (15:1, v/v) solution was added. The mixture was then centrifuged at 2236 x g for 15 min following which the upper layer was separated, and the intensity of the resulting pink color was read at 532 nm. Tetramethoxypropane was used as an external standard and the level of lipid peroxides was expressed as nmol of MDA formed/g wet weight.
Estimation of Nitric Oxide Generation in Eye Lens
Generation of NO in the eye lens of Wistar rat pups was determined by the method of Ozbek et al. 61 with slight modifications. For this assay, 100 μl of clear eye lens homogenate supernatant obtained from the various experimental groups was mixed with 150 μl Tris-HCl buffer (pH 7.4). It was incubated with 5 μl of 0.01 U nitrate reductase and 10 μl of 2 mM β-NADH for 20 min at 22°C in the dark with constant shaking. After this, 50 μl of 1% sulfanilamide followed by 50 μl of 0.1% naphthylethylenediamine dihydrochloride was added and incubated for 10 min at room temperature. At the end of incubation, the samples were centrifuged (12,000 rpm, 15 min, 4°C) to pellet any precipitate that may have formed and the absorbance of the clear supernatant was read at 540 nm in a Shimadzu (UV-160 A) spectrophotometer against a reagent blank consisting of buffer and Griess reagent. The nitrite (=NO) generated in the eye lens was determined against sodium nitrite in a standard curve and the amount of nitrite was expressed as μM nitrite.
Estimation of Hydroxyl Radical Generation in the Eye Lens
The generation of OH· in the eye lens of Wistar rat pups was detected spectrophotometrically by Halliwell and Gutteridge 62. Briefly, eye lens obtained from the various experimental groups was incubated in 700 μl phosphate buffer (pH 7.8, 0.1 M EDTA), 2 mM sodium salicylate, and 40 μl 10 N HCl and to this 0.25 g of NaCl was added. To this mixture an equal volume of chilled diethyl ether was added and incubated for 30 min at 25°C. The absorbance was read at 510 nm in a UV-160 A Shimadzu spectrophotometer against a suitable reagent blank and the OH· generation was expressed as absorbance at 510 nm/30 min.
Intracellular Calcium Analysis
Total lens calcium was estimated by the method of Inomata et al. 63. The dry weight of lenses was measured after heating at 100°C for 20 h. The lenses were extracted with 0.2 ml of concentrated HCl at room temperature overnight and made up to 1.0 ml with deionized water. The mixtures were then centrifuged at 10,000 rpm for 10 min to remove insoluble material and calcium concentration in the supernatant fraction was measured by an atomic absorption spectrophotometer.
Estimation of Lens ATPase: Ca2+, Na+/K+, and Mg2+ ATPases
Ca2+ ATPase activity was assayed according to the method of Hjerten and Pan 64. This involves 10 mM ATP as substrate in the presence of Tris-HCl buffer (125 mM, pH 8.0) containing 50 mM calcium chloride. Lens Na+/K+ ATPase activity was estimated by the method of Bonting 65. The reaction mixture contained 40 mM ATP as substrate and Tris–HCl buffer (90 mM, pH 7.5) containing 50 mM magnesium sulfate, 50 mM potassium chloride, 600 mM sodium chloride and 1 mM EDTA. Mg2+ ATPase activity in eye lens was estimated by the method of Ohnishi et al. 66, in a reaction containing 0.01 M ATP and Tris-HCl buffer (375 mM, pH 7.6) containing 60 mM magnesium chloride.
Western Blot Analysis
The whole lenses from each group were homogenized in 10 volumes (v/w) of 20 mM Tris-HCl (pH 7.4) containing 5 mM EDTA and 10 mM mercaptoethanol using a glass–glass Dounce homogenizer. The homogenates were centrifuged at 12,000 rpm, 30 min, 4°C and the recovered supernatants were used as lens protein. The lens proteins prepared from normal and treated rat pups were electrophoresed by the method of Laemmli, 67 in 12% SDS-PAGE slab gels. After electrophoretic separation, the proteins were transferred onto Immobilon nitrocellulose membranes. Immunoblotting was performed with rabbit and rat polyclonal primary antibodies, i.e., anti- p65 subunit of NF-κB, anti-iNOS and anti- αA-, αB-crystallin respectively (at a dilution of 1:500 each), after blocking with non-fat dry milk powder. This was later incubated with peroxidase-tagged goat anti-rabbit IgG secondary antibody, and immune complexes were detected using DAB (0.01%) as well as H2O2.
Reversed Transcription-polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from normal and cataract lenses using the acid guanidium thiocyanate-phenol-chloroform extraction method by Chomcynski and Sacchi, 68 with Trizol reagent (Sigma) according to the manufacturer's instructions. Oligo(dT)-primed first-strand cDNA was prepared from total lens RNA using AMV reverse transcriptase at 37°C for 60 min. PCR was performed with gene-specific primers using Taq DNA polymerase (Bangalore Genei). The primers used for iNOS were based on the rat sequence of Inomata et al. 27, 5′-GCCTCCCTCTGGAAGA-3′ (sense) and 5′-TCCATGCAGACAACCTT-3′ (antisense). The primers used for αA-, αB-crystallin and Hsp 70 were also based on the rat sequences 5′-CACCGTGAAGGTACTGGAAG-3′ (sense), 5′- TCAGGAAGGCAGACTCTTG-3′ (antisense), 5′-AGA GCA CCT GTT GGA GTC TG-3′(sense), 5′-TTC CTT GGT CCA TTC ACA GT-3′ (antisense), 5′-ATG AAG GAG ATC GCC GAG G-3′ (sense), and 5′- GTC GAA GAT GAG CAC GTT G-3′ (antisense) respectively. The primers for β-actin as the reference control were 5′-GTGGCCGCTCTAGGCACCA-3′ and 5′CGGTTGGCCTTAGGGTTCAGGGGGG-3′. The following cycling conditions were used: 120 s of initial denaturation at 94°C followed by 30 cycles of 90 s at 94°C, 60 s at 60°C, and 60 s at 72°C, followed by 5 min at 72°C. The amplification products were electrophoresed on an agarose gel (2%) in Tris-acetate EDTA buffer (pH 8.2). Bands, stained by ethidium bromide, were visualized by a UV-trans illuminator (Bio-Rad Gel Doc system).
Cytochemical Localization of Nitroblue Tetrazolium (NBT)-Reducing Substances in the Eye Lens
The generation of O2- in the eye lens of Wistar rat pups was detected cytochemically using NBT reduction method by Manikandan et al. 69. Briefly, the whole eye lens was incubated with 100 μl of 0.3% NBT for 1 h at 22°C. After incubation, the eye lenses obtained from various experimental groups were washed with Tris-HCl buffer (pH 7.4, 0.1M Tris). The eye lenses were then examined for blue formazan deposits under bright-field optics (4×) using Carl Zeiss Axiolab microscope.
Immunohistochemical Analysis for iNOS and αA- & αB– Crystallin Expression
Immunohistochemistry was carried out by the method of Yao et al. 70 on 5-μm paraffin-embedded tissue sections on poly-L-lysine-coated glass slides. The tissue sections were deparaffinized by placing the slides in an oven at 60°C for 10 min and then rinsed twice in xylene for 10 min each. The slides were then hydrated in a graded ethanol series (100, 90, 70, 50, 30% for 10 min each) and then finally in double-distilled water for 10 min. The sections were incubated with 1% H2O2 in double-distilled water for 15 min at 22°C, to quench endogenous peroxidase activity. The sections were rinsed with Tris-HCl containing 150 mM NaCl (pH 7.4) and blocked in blocking buffer (tris-buffered saline (TBS), 0.05% Tween, 5% non-fat dry milk (NFDM)) for 1 h at 22°C. After being washed with TBS containing 0.05% Tween 20, the sections were incubated with primary antibodies, anti- αA- and αB-crystallin, as well as anti-iNOS polyclonal rabbit antibody, at a dilution of 1:500 each overnight at 4°C. After incubation the eye lens sections were rinsed with TBS containing 0.05% Tween 20 twice and incubated with secondary antibody, goat anti-rabbit IgG-HRP conjugate, at a dilution of 1:3000, for 1 h at 4°C. After another wash with TBS containing 0.05% Tween 20, the immunoreactivity was developed with 0.05% diaminobenzidine (DAB) and 0.01% H2O2 for 1-3 min. The eye lens sections were observed (4×) for brown color formation under bright field using a Carl Zeiss Axioscope microscope.
Immunofluorescence Expression of p65/NF-κB
Deparaffinized tissue sections were rehydrated and blocked in 5% BSA in TBS with 0.05% Tween 20. Sections were then incubated overnight with anti-p65/ NF-κB (at a dilution of 1: 500) and then with secondary antibody conjugated to FTC (at 1: 40 dilution) in the dark at room temperature for about 2 h. The sections were then counter-stained with either PI or DAPI and visualized under a laser scanning confocal microscope (LSM 10).
Statistical Analysis
All results are expressed as mean ± SD. Independent sample student’s t test was used to assess the data. A p > 0.05 was considered statistically significant.
Results
Resveratol Lowers Selenite Induced MDA Levels in Both Lens and Serum of Treated Rat Pups
Malondialdehyde (MDA) is a major reactive carbonyl product that is well known to cause cell damage arising from oxidative stress. We observed significant elevation in the level of MDA (Figure 1) in group II treated rat pups that are significantly down-regulated in reseveratol treated groups III and V.
Figure 1.Malondialdehyde level in serum (dark shaded bars) and eye lens (light shaded bars) from different experimental group animals. Each value represents mean ± SD of 4 determinations using samples from different preparations. The difference in MDA levels observed between groups I & II and groups II & III-V animals were statistically significant at * p < 0.05.
Resveratrol Treatment Down-Regulates Nitric Oxide and Hydroxyl Radical Generation Induced by Sodium Selenite
The generation of free radicals is a characteristic feature in mounting cellular oxidative insult. Two major mediators in this regard, nitric oxide and hydroxyl radical, have been known to play vital roles in promoting cataract formation. In this regard, the inhibition in both these mediators upon selenite treatment (Figure 2 and Figure 3) was observed to be suppressed by resveratrol (groups III and V) and this was found to be statistically significant.
Figure 2.Nitric oxide level in the eye lens from different experimental group animals. Each value represents mean ± SD of 4 determinations using samples from different preparations. The difference in nitric oxide levels observed between groups I & II and groups II & III-V animals were statistically significant at * p < 0.05.
Figure 3.Hydroxyl radical level in from different experimental group animals. Each value represents mean ± SD of 4 determinations using samples from different preparations. The difference in hydroxyl radical levels observed between groups I & II and groups II & III-V animals were statistically significant at * p < 0.05.
Selenite Perturbs Total Lens calcium Level and Intracellular Ca2+ATPase Activity that is Regulated by Resveratrol Treatment
In further corroboration to the purported effects of resveratrol on intracellular calcium regulation, we observed a significant elevation in intracellular calcium levels in lens tissue in group II that was lowered in groups III and V when compared to untreated rats (Figure 4). This can be attributed to the aberration in intracellular calcium levels in selenite treated pups including disruption in Ca2+ ATPase activity as shown in Figure 5. However, resveratrol did not show any significant modulation to the activities of cellular Na+/K+ and Mg2+ ATPases (Data not shown). This implies that it is lens Ca2+ that is probably dysregulated during the pathophysiology of cataract formation.
Figure 4.Total calcium levels in the eye lens from different experimental group animals. Each value represents mean ± SD of 4 determinations using samples from different preparations. The difference in total calcium levels observed between groups I & II and groups II & III-V animals were statistically significant at * p < 0.05.
Figure 5.Calcium ATPase activity in the eye lens from from different experimental group animals. Each value represents mean ± SD of 4 determinations using samples from different preparations. The difference in Ca2+ ATPase activity observed between groups I & II and groups II & III-V animals were statistically significant at * p < 0.05.
Resveratrol Replenishes Cellular Enzymic and Non-enzymic Antioxidants
The levels of cellular enzymatic and non-enzymatic antioxidants were assayed among the different experimental groups. Upon analysis, it was revealed that sodium selenite exerted significant inhibition on all cellular antioxidants assayed when compared to controls (Table 1 and Table 2). Interestingly, lens samples from groups III and V displayed an improvement in the levels of such cellular anti-oxidants that was also statistically significant when compared to group I.
Table 1. Assay for enzymic antioxidant levels in serum and eye lens from the different experimental groups.| Enzymes analyzed (Unit of activity) | Group I | Group II | Group III | Group IV | Group V |
| Catalase (mmol H 2 O 2 consumed/mg protein/min) | |||||
| Serum | 19.46 ± 1.66 | 11.97 ± 0.92* | 20.26 ± 1.28* | 12.00 ± 1.18 | 19.71 ± 1.83* |
| Eye lens | 6.34 ± 1.02 | 4.33 ± 0.59* | 6.31 ± 1.51* | 4.68 ± 1.20 | 6.89 ± 1.82* |
| Superoxide Dismutase (50 % inhibition of adrenaline auto oxidation/min) | |||||
|---|---|---|---|---|---|
| Serum | 16.42 ± 2.32 | 11.14 ± 1.60* | 16.35 ± 2.22* | 11.67 ± 1.33 | 15.28 ± 1.60* |
| Eye Lens | 3.69 ± 0.51 | 2.26 ± 0.52* | 3.35 ± 1.03* | 2.57 ± 0.48 | 3.47 ± 0.59* |
| Glutathione peroxidase (mmol glutathione oxidized/ mg protein/min) | |||||
| Serum | 1.91 ± 0.20 | 1.04 ± 0.15* | 1.85 ± 0.20* | 1.02 ± 0.13 | 1.07 ± 0.30* |
| Eye lens | 1.81 ± 0.16 | 0.88 ± 0.11* | 1.49 ± 0.25* | 0.89 ± 0.24 | 1.40 ± 0.29* |
| Glutathione-S-transferase ( μmol of CDNB conjugated/min/mg/protein) | |||||
| Serum | 4.0 ± 0.23 | 2.33 ± 0.48* | 3.36 ± 0.57* | 2.42 ± 0.56 | 3.11 ± 0.57* |
| Eye lens | 80.50 ± 2.08 | 64.44 ± 5.91* | 75.59 ± 5.57* | 65.89 ± 4.83 | 73.11 ± 4.80* |
Gene and Protein Expression Levels of Cellular Stress Mediators are Modulated by Resveratrol
Cell stress mediators including the heat shock protein Hsp 70, lens - αA-, αB-crystallins, iNOS and p65/NF-κB were analysed both for their relative gene and protein expression levels among all the different experimental groups. The expression was compared with the untreated group and normalized to the house keeping gene, β- actin.
RT-PCR results revealed an up-regulation in Hsp 70, lens αA- and αB-crystallins and iNOS levels in group II (Figure 6) that was reduced with resveratrol administration in groups III and V. The protein level expression for these molecular chaperones also mimicked those obtained by us earlier with gene expression study. Upon Western analysis, it was revealed that inducible nitric oxide synthase (iNOS), p65/NF-κB and αA- and αB-crystallins (Figure 7) were significantly elevated in group II rats, while such expression was found to be decreased upon resveratrol pre- or co-treatments (groups III and V).
Figure 6.RT PCR analysis for Hsp 70, iNOS, αA and αB crystallin gene expression in the eye lens from different experimental group animals. Reverse transcribed cDNA isolated from- Lane I: control (group I); lane II selenite only (group II); lane III: selenium + resveratrol simultaneously (group III); lane IV: Resveratrol administered 24 h after treatment with selenite (group IV); lane V: Resveratrol administered 24 h before treatment with selenite (group V).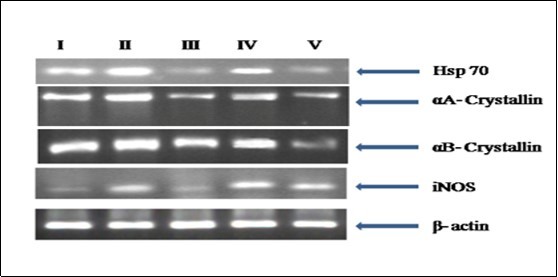
Figure 7.Western blot analyis for p65/NF-kB, iNOS, αA and αB crystallin protein expression in the eye lens from different experimental group animals. Lens homogenates from from- Lane I: control (group I); lane II selenite only (group II); lane III: selenium + resveratrol simultaneously (group III); lane IV: Resveratrol administered 24 h after treatment with selenite (group IV); lane V: Resveratrol administered 24 h before treatment with selenite (group V). ). Separated lens protein was pre-incubated with anti-iNOS, p56/NF- kB and αA, αB crystallin polyclonal rabbit antibody (1: 500 dilution) and subsequently with goat anti-rabbit IgG-HRP (1: 2500 dilution). The immunoreactivity was developed with 0.01% DAB and H2O2.
Resveratrol Reduces Selenite Mediated Superoxide Anion Generation and Formazan Deposition in Lens Tissue
The generation of potent free radical mediators was assayed via the NBT method wherein the production of superoxide anion was positively correlated with group II lens homogenates (Figure 8). This also reflected in increased deposition of the deep blue formazan deposits in the whole lens from the same group (group II; Figure 8 B). In contrast, both lens and lenticular homogenates from groups III and V (Figure 8 C and E) demonstrated a significant reduction in superoxide anion generation as well as formazan deposition indicative of cytoprotective potential of resveratrol. The results of the NBT assay were also observed by the cytochrome C reduction method (Table 3) with higher reduction observed with group II rats, whereas reduction was found to be lower in group III and V rats. This indicates higher levels of free radical generation in group II rats when compared to resveratrol administered rats.
Figure 8.NBT reduction in the eye lens of from different experimental group animals to assay for superoxide anion generation. Blue colour formation upon incubation with 0.3% NBT for observed after 45 min with intense blue colour formation indicative of superoxide anion generation. Inset (A): Physiological saline treated (group I); (B) Selenite treated (group II); (C) Selenium and resveratrol administered simultaneously (group III); (D) Resveratrol administered 24 h after treatment with selenite (group IV); (E) Resveratrol administered 24 h before treatment with selenite (group V).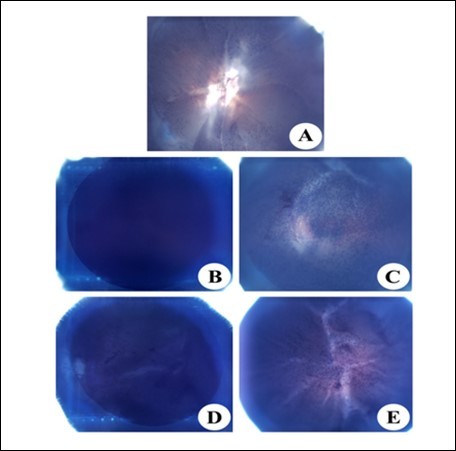
| Test condition | Cytochrome C reduction (OD 550 nm/ 15 min) |
| Control (group I) | 0.312 ± 0.026 |
| Selenite only (group II) | 0.560 ± 0.054* |
| Selenite + SOD (65 units) | 0.458 ± 0.045 |
| Selenite + resveratrol administered simultaneously (group III) | 0.318 ± 0.041* |
| Resveratrol gavaged 24 h prior to Selenite treatment (group IV) | 0.584 ± 0.079 |
| Resveratrol 24 h post selenite treatment (group V) | 0.477 ± 0.072* |
Resveratrol Diminishes iNOS and αA-, αB-crystallin Protein Levels
The expression of iNOS and αA- and αB-crystallins in the lens sections from the different experimental groups was studied via immunohistochemical analysis. Group II lens exhibited markedly a higher degree of staining for iNOS and crystallin proteins (Figure 9 and Figure 10). Further, immunohistochemical analysis of lenses groups III and V showed that the staining was minimal and the sections were comparable to the lens from control group.
Figure 9.Immunohistochemical analysis of iNOS in the eye lens from different experimental group animals. Lens sections were preincubated with anti-iNOS polyclonal rabbit antibody (1: 500 dilution) and subsequently with goat anti-rabbit IgG-HRP (1: 3000 dilution). The immunoreactivity was developed with 0.01% DAB and H2O2. Note brown color formation indicative of peroxidase reaction in the nucleus. Inset (A): Physiological saline treated (group I); (B) Selenite treated (group II); (C) Selenium and resveratrol administered simultaneously (group III); (D) Resveratrol administered 24 h after treatment with selenite (group IV); (E) Resveratrol administered 24 h before treatment with selenite (group V).
Figure 10.Immunohistochemistry of αA and αB crystallin in the eye lens from different experimental group animals. Lens sections were preincubated with anti- αA, αB crystallin polyclonal rabbit antibody (1: 500 dilution) and subsequently with goat anti-rabbit IgG-HRP (1: 3000 dilution). The immunoreactivity was developed with 0.01% DAB and H2O2. Note the brown colour formation that is indicative of peroxidase reaction in the nucleus. Inset (A): Physiological saline treated (group I); (B) Selenite treated (group II); (C) Selenium and resveratrol administered simultaneously (group III); (D) Resveratrol administered 24 h after treatment with selenite (group IV); (E) Resveratrol administered 24 h before treatment with selenite (group V).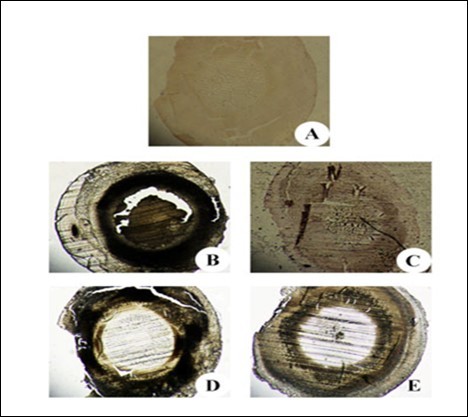
Resveratrol Down-regulates p65/NF-κB Expression
An attempt was also made to analyse the expression of p65/NF-κB and relate it to late stage apoptotic cells (propidium iodide staining) by immunofluorescence in isolated rat lenses from different treatment groups. As can be seen from the results (Figure 11), the co-expression level of both p65/NF-κB and apoptosis marker propidium iodide was found to be higher in lenses from group II rats, when compared to controls. However, such expression was minimal in the case of resveratrol administered rat lenses (groups III and V), indicating the ability of resveratrol in preventing NF-κB induced inflammatory changes.
Figure 11.Immunofluorescence analysis for the expression of NF-kB in the eye lens from different experimental group animals. Lens section were pre-incubated with anti-p65/ NF-κB (at a dilution of 1: 500) and then with secondary antibody conjugated to FITC (at 1: 40 dilution) in the dark at room temperature for about 2 h. The sections were then counter-stained with either PI or DAPI. Please note the sub-insets i, ii, iii and iv in each of the insets indicate staining with DAPI (in blue), NF-kB (in green), PI (Red) and merged images respectively. The expression levels of NF-kB and PI were observed to be higher in group II in comparison to groups I, III and V. Inset (A): Physiological saline treated (group I); (B) Selenite treated (group II); (C) Selenium and resveratrol administered simultaneously (group III); (D) Resveratrol administered 24 h after treatment with selenite (group IV); (E) Resveratrol administered 24 h before treatment with selenite (group V).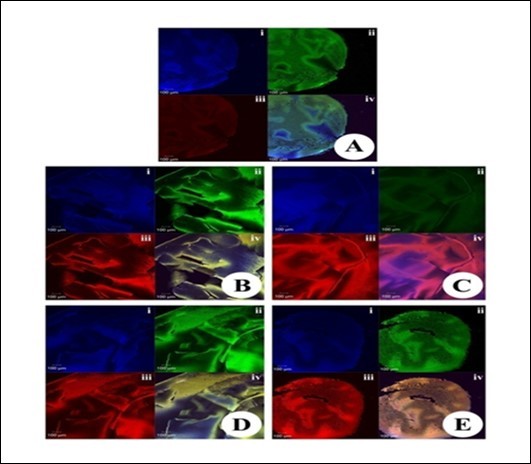
Discussion
The anterior lens epithelium and it underlying fibre cell mass are of utmost importance in the regulation of calcium level and its transport which has been highlighted in several different studies. For instance, Rhodes and Sanderson 71 reported that lens epithelium and fibre cells concentration of calcium to be in minuscule amounts ranging from 100 nM and 10 µM respectively under normal conditions. Failure to maintain this extremely low level (as seen with cataract lenses reaching 900 µM concentrations) exposes the lens to calcium mediated damage. It is in this light we investigated both the intracellular calcium levels in lens tissue besides the functioning of the Ca2+ ATPase. As expected, treatment with sodium selenite (group II) resulted in both an increase in intracellular calcium levels and a reduction in the Ca2+ ATPase activity (Figure 4 and Figure 5) that was mitigated by treatment with resveratrol in groups III and V. A direct effect to elevated calcium load in lens tissue leads to calpain activation and consequently the lens crystallin degradation, a characteristic feature in cataract formation.
The lens crystallin proteins are the major structural proteins that maintain lens transparency, which is a principal function. The α-crystallin is known to prevent protein aggregation and functions as a molecular chaperone that is especially important during conditions of cellular stress. This ability is progressively compromised in aging lens tissue and is the prime target of calpain mediated truncation (due to elevated intracellular calcium levels) that affects its structure and solubility (increased cross-linking of subunits results in lower chaperone activity 72. This overwhelms the ability of lens to overcome protein aggregation including the β-, γ-crystallins and other cytoskeletal proteins that lead to light refraction as well as eventually clouding of the lens tissue. We therefore sought to shed light in the fate of crystallins both at the gene expression and protein regulation across the different experimental groups. The gene expression levels of cell stress mediators including heat shock protein Hsp 70, lens - αA-, αB-crystallins and iNOS were markedly upregulated by treatment with sodium selenite (group II) and mitigated with resveratrol administration (again in groups III and V that was comparable with group I; Figure 6). A similar trend in the upregulation of iNOS and αA-, αB- crystallin proteins were observed in group II treated lenses that were down-regulated with resveratol treatment (Figure 7). We would also like to bring attention to the major transcription factor that regulates cell inflammatory signaling, NF-κB, and probing for the p65 subunit revealed an increase in the level of this protein in group II lenses that was down-regulated in those administered with resveratrol (groups III and V that match untreated lenses from group I).
The ocular lens tissue is subjected to a milieu of oxidants including photo-oxidative stress inducers. Metabolic stress to mitochondria as a result of any imbalance in the cell’s redox status can pave the way for induction of apoptosis via different mechanisms. Páramoa et al. 73 for instance reported glucose deprivation leads to oxidative stress and in turn activation of calpain via elevated calcium levels that can trigger apoptosis. An elevated and sustained generation of free radicals has been known to damage cellular components that include those that regulate intracellular calcium levels 74. It was therefore important in our study we assessed the levels of major antioxidants and also prominent free radicals in addition to malondialdehyde (MDA), a well known marker for oxidative stress, to get a complete picture of the lens redox status from different experimental groups. Nearly a two-fold increase in both the levels of MDA, hydroxyl radical and at least a three-fold elevation in nitrite levels were recorded in group II lenses that were significantly reduced in groups III and V (Figure 1). Similarly, the assays for cellular antioxidant enzymes revealed the loss of activity including catalase, SOD, glutathione peroxidase and glutathione S-transferase in the sodium selenite treated group II pups both in serum and lenticular tissue. This was significantly abrogated upon treatment with resveratrol wherein a comparable improvement in the levels of all assayed antioxidant enzymes undertaken here was observed (Table 1). We also report a similar pattern that was exhibited in the assay for reduced glutathione level across the different experimental groups (Table 2).
In our continued investigation into the cellular oxidants, we also undertook the NBT cytochemical detection test to identify the localization of superoxide anion (O2-), an important cellular oxidant that can form the highly reactive peroxynitrite anion (ONOO-) in the presence of NO. The cell damage caused here can be extensive including the impairment in mitochondrial function, generation of other free radical intermediates and cellular protein nitration 75. As observable in Figure 8, the intense NBT staining in the lens central core in group II was typical of sodium selenite induced oxidative stress that was ameliorated in lenses from groups III and V.
Dietary polyphenols have been known to possess numerous beneficial effects including their potential in regulating inflammation. The nicotinamide adenine dinucleotide (NAD+) dependent family of enzymes, the sirtuins, are a known target of the polyphenol, resveratrol 76. Sirtuins carry out a variety of cellular functions including maintenance of cellular homeostasis, regulating the cell cycle, apoptosis, DNA repair and also in cellular stress signaling mechanisms. The major transcription factor, NF-κB, is widely known to promote pro-inflammatory responses including those such as iNOS, TNF-α, IL-8, MCP-1 and IL1β and resveratrol is known to regulate NF-κB expression 77. Only very few reports exist that investigate the anti-cataract effects of resveratrol. The sodium selenite model of cataract formation in rat pups has been well established and is believed to closely resemble the age related cataract development in human that is typified with heightened oxidative stress, aberrations in intracellular calcium and protein aggregation. One such investigation by Doganay et al. 78 demonstrated the potential of resveratol to mitigate cataract through restoring antioxidant defense systems and also reduce the level of malondialdehyde. The focus will need to be devising novel therapies using phyto-compounds such as resveratrol that could both be effective and also avoid the unwanted side-effects of currently available drugs such as the NSAIDs (non-steroidal anti inflammatory drugs).
Conclusion
The result concluded that the cytosolic level of calcium is tightly regulated under normal conditions. The pro-oxidant effects of sodium selenite have been established and elevated levels of free radicals can lead to cell damage including cellular mechanisms that regulate intracellular calcium levels. This ultimately leads to mitochondrial dysfunction, activation of pro-inflammatory signalling and activation of the cell death cascade. We postulate, using the sodium selenite induced Wistar rat pup model of cataractogenesis, that administration of resveratrol restores the redox balance disrupted by selenite in addition to modulating the levels of lens crystallin proteins and p65/NF-κB to alleviate cataract formation.
References
- 1.Watkins R. (2002) Foundations of a solution to cataract blindness. , Clinical Experimental Optometry 85, 59-60.
- 2.Brian G, Taylor H. (2001) Cataract blindness-challenges for the 21st century.Bulletin of the World Health Organization,79. 249-256.
- 3.V B Gupta, Rajagopala M, Ravishankar B. (2014) Etiopathogenesis of cataract: An appraisal.Indian. , J.Ophthalmol 62(2), 103-110.
- 4.Spector A. (1995) Oxidative stress-induced cataract: mechanism of action.FASEB. , Journal 9, 1173-1182.
- 5.Taylor A, K J Davies. (1987) Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens.Free Radical Biology Medicine. 3, 371-377.
- 6.Taylor A, P F Jaques, C K Dorey. (1993) Oxidation and aging: impact on vision.Toxicology. , Industrial Health 9, 349-371.
- 7.Haberal M, Hamaloglu E, Bora S, Oner G, Bilgin N. (1988) The effects of vitamin E on immune regulation after thermal injury.Burns. , Including Thermal Injury 14, 388-393.
- 8.K F Gey, Puska P, Jordan P, U K Moser. (1991) Inverse correlation between plasma vitamin E and mortality from ischemic heart disease in cross-cultural epidemiology.American. , Journal of Clinical Nutrition 53, 326-334.
- 9.Bankson D D, Kestin M, Rifai N. (1993) Role of free radicals. in cancer and atherosclerosis.Clinics Laboratory Medicine,13 463-480.
- 10.Dogru M, Kojima T, Simsek C, Tsubota K. (2018) Potential role of oxidative stress in ocular surface inflammation and dry eye disease.Invest.Ophthalmol.Visua. , Sci 59, 163-168.
- 11.Spectore A, Li S, Sigelman J. (1974) Age dependent changes in the molecular size of human lens proteins and their relationship to light scatter.Investigative. , Ophthalmology Visual Science 13, 795-798.
- 12.S D Varma, N A Beachy, R D. (1982) Photoperoxidation of lens lipids: prevention by vitamin E.Photochemistry and. , Photobiology 36, 623-626.
- 13.Graham A, Hogg N, Kalyanaraman B, O'Leary V, Darley-Usmar V et al. (1993) Peroxynitrite modification of low-density lipoprotein leads to recognition by the macrophage scavenger receptor.FEBS. , Letter 330, 181-185.
- 14.Hofseth L J, Robles A I, Espey M G, Harris C C. (2005) Nitric oxide is a signaling molecule that regulates gene expression.Methods Enzymology. 396, 326-340.
- 15.Sumi D, L J Ignarro. (2004) Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide.Diabetes. 53, 1841-1850.
- 16.Zhang H, Chen X, Teng X, Snead C, J D Catravas. (1998) Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells.Biochemistry. , Pharmacology 55, 1873-1880.
- 17.C J Lowenstein, E W Alley, Raval P, A M Snowman, S H et al. (1993) Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide.Proceeding of the National Academy of Science. , USA 90, 9730-9734.
- 18.Q W Xie, Kashiwabara Y, Nathan C. (1994) Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase.Journal of Biology Chemistry. 269, 4705-4708.
- 19.K F Beck, R B Sterzel. (1996) Cloning and sequencing of the proximal promoter of the rat iNOS gene: activation of NF kappa B is not sufficient for transcription of the iNOS gene in rat mesangial cells.FEBS. Letter,394 263-267.
- 20.Vera M E de, R A Shapiro, A K Nussler, J S Mudgett, R L Simmons et al. (1996) Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. , Proc. Natl. Acad. Sci. USA 93, 1054-1059.
- 21.Jia J, Liu Y, Zhang X, Liu X, Qi J. (2013) Regulation of iNOS expression by NF-kB in human lens epithelial cells treated with high levels of glucose.Invest.Ophthalmol.Visua. , Sci 54, 5070-5077.
- 22.H Y Yun, V L Dawson, T M Dawson. (1997) Nitric oxide in health and disease of the nervous system.Molecular. , Psychiatry 2, 300-310.
- 24.Brune B, A von Knethen, K B Sandau. (1998) Nitric oxide and its role in apoptosis.European. , Journal Pharmacology 351, 261-272.
- 25.Becquet F, Courtois Y, Goureau O. (1997) Nitric oxide in the eye: multifaceted roles and diverse outcomes.Survey. , Ophthalmology 42, 71-82.
- 26.G C Chiou. (2001) Review: effects of nitric oxide on eye diseases and their treatment.Journal of Ocular Pharmacology Therapeutics,17. 189-198.
- 27.Inomata M, Hayashi M, Shumiya S, Kawashima S, Ito Y. (2001) Involvement of inducible nitric oxide synthase in cataract formation in Shumiya cataract rat (SCR).Current. , Eye Research 23, 307-311.
- 28.Ito Y, Nabekura T, Takeda M, Nakao M, Terao M et al. (2001) Nitric oxide participates in cataract development in selenite-treated rats.Current. , Eye Research 22, 215-220.
- 29.Shearer T R, David L L. (1983) Role of calcium in selenium cataract.Current. , Eye Research 2, 777.
- 30.Velpandian T, Nirmal J, Gupta P, Vijayakumar A R, Ghose S. (2010) Evaluation of calcium dobesilate for its anti-cataract potential in experimental animal models.Methods. , Find. Exp. Clin.Pharmacol,32 3, 171-179.
- 31.David L L, Shearer T R. (1984) State of sulfhydryl in selenite cataract.Toxicology and. , Applied Pharmacology 74, 109.
- 32.K R Hightower, McCready J. (1991) Effect of thiol reagents on Ca-ATPase in rabbit lens epithelium.Current. , Eye Research 10, 299-303.
- 33.P L Bergad, W B Rathbun. (1986) Inhibition of Na-K-ATPase by sodium selenite and reversal by glutathione.Current Eye. Research,5 919-923.
- 34.Tomlinson J, S C Bannister, P C Croghan, Duncan G. (1991) Analysis of rat lens 45 Ca2+fluxes: evidence for. , Na+– Ca2+exchange.Experimental Eye Research 52, 619-627.
- 35.Wang Z, J L Hess, G E Bunce. (1992) Calcium efflux in rat lens: Na+/Ca2+exchangerelated to cataract induced by selenite.Current. , EyeReseach 11, 625-632.
- 36.Liu K, Lyu L, Chi D, Gao J, Sun X et al. (2015) Altered ubiquitin causes perturbed calcium homeostasis, hyperactivation of calpain, dysregulated differentiation and cataract. , Proc. Natl. Acad. Sci. USA 112(4), 1071-1076.
- 37.Suzuki K, T C Saido, Hirai S. (1992) Modulation of cellular signals by calpain.Annals of the New York. , Academy of Science 674, 218-227.
- 38.T C Saido, Nagao S, Shiramine M, Tsukaguchi M, Yoshizawa T et al. (1994) Distinct kinetics of subunit autolysis in mammalian m-calpain activation.FEBS. , Letter 346, 263-267.
- 39.Awasthi S, S K, J T Piper, S, Chaubey M et al. (1996) Curcumin protects against 4-hydroxyl-2-trans nonenal-induced cataract formation in rat lenses.American. , Journal Clinical Nutrition,64 761-766.
- 40.S K Gupta, Srivastava S, Trivedi D, Joshi S, Halder N. (2005) Ocimum sanctum modulates selenite-induced cataractogenic changes and prevents rat lens opacification.Current. , Eye Research 30, 583-591.
- 41.Maddirala Y, Tobwala S, Karacal H, Ercal N. (2017) Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in wistar rats.BMCOphthamology,17. 54.
- 42.Gusman J, Malonne H, Atassi G. (2001) A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. , Carcinogenesis 22(8), 1111-1117.
- 43.El-Mowafy A M, Alhalaf M. (2003) Resveratrol activates adenylyl-cyclase in human breast cancer cells: a novel, estrogen receptor-independent cytostatic mechanism.Carcinogenesis. 24(5), 869-873.
- 44.S H Roberts, Metin A. (2001) Inhibition of protein kinase C by resveratrol.Biochemical. , Pharmacology 62, 1647-1651.
- 45.E N Frankel, A L Waterhouse, J K Kinsella. (1993) Inhibition of human LDL oxidation by resveratrol.Lance. 24(341), 1103-1104.
- 46.Creasy L L, Coffee M. (1988) Phytoalexin production potential of grape. , berries.Journal of American Society for Horticulture Science 113, 230-234.
- 47.Chanvitayapongs S, Draczynska-Lusiak B, A Y Sun. (1997) of oxidative stress by antioxidants and resveratrol. in PC12 cells. Neuroreport.8: 1499-1502.
- 48.Belguendouz L, Fremont.L.,Linard, A.(1997).Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins.Biochemical Pharmacology,53,1347-1355.
- 49.Celotti E, Ferrarini R, Zironi R, L S Conte. (1996) content of some wines obtained from dried Valpolicella grapes : Recioto and Amarone.Journal. 730-47.
- 50.Jang M, Cai L, G O Udeani, K V Slowing, C F Thomas et al. (1997) chemo preventative activity of resveratrol, a natural product derived from grapes. Science.275: 218-220.
- 51.G J Soleas, E P Diamandis, D M Goldberg. (1997) a molecule whose time has come ? and gone ?Clinical Biochemistry,30,91-113.
- 52.Ostadalova I, Babicky A, Obenberger J. (1978) Cataract induced by administration of a single dose of sodium selenite to suckling rats.Experientia. 34, 222-223.
- 53.O H Lowry, N J Rusebrough, A L Farr. (1951) . Protein measurement with folinphenol reagent.Journal Biological Chemistry,193 265-275.
- 54.R F Beers, I W Sizer. (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase.Journal. , Biological Chemistry 195, 133-140.
- 55.H P Misra, Fridovich I. (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase.Journal of. , Biological Chemistry 247, 3170-3175.
- 56.J T Rotruck, A L Pope, H F Ganther. (1973) Selenium: Biochemical role as a component of glutathione peroxidase.Science. 179, 588-590.
- 57.W H Habig, M J Pabst, B W Jacoby. (1974) Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation.Journal. , Biological Chemistry 249, 1730-1737.
- 58.Moron M S, Depierre J W, Mannervik B. (1979) Levels of glutathione, glutathione reductase and glutathione-S-transferaseactivities in rat lung and liver.BiochimicaetBiophysicaActa,582. 67-68.
- 59.K C Bhuyan, D K Bhuyan, Santos O, S M Podos. (1992) Antioxidant and anticataractogenic effects of topical captopril in diquat-induced cataract. in rabbits.Free Radical Biological Medicine,12 251-261.
- 60.Ohkawa H, Ohishi N, Yagi K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.Analytical. , Biochemistry 95, 351-358.
- 61.Ozbek E, Turkoz Y, Gokdeniz R. (2000) Increased nitric oxide production in the spermatic vein of patients with varicocele.European Urology. 37, 172-175.
- 62.Halliwell B, Gutteridge J M C. (1986) Hydroxyl radicals assayed by aromatic hydroxylation and deoxyribose degradation.In:Greenwald,R.A.(Ed.),Handbook of Methods for Oxygen Radical Research. , Boca Raton 177-180.
- 63.Inomata M, Nomura K, Takehana M, T C Saido, Kawashima S et al. (1997) Evidence for the involvement of calpain in cataractogenesis in Shumiya cataract rat (SCR).BiochimicaetBiophysicaActa,1362. 11-23.
- 64.Hjerten S, Pan H. (1983) Purification and characterization of two forms of low-affinity of Ca2+- ATPases from erythrocyte membrane.BiochimicaetBiophysicaActa,728. 281-288.
- 65.S L Bonting. (1970) Sodium-potassium activated adenosine triphosphate and cation transport. , In: Bittar, E.E. (Ed.), Membrane and Ion Transport, Vol. I. Wiley-Interscience, London,pp 257-363.
- 66.Ohnishi T, Suzuki T, Suzuki Y, Ozawa K. (1982) A comparative study of plasma membrane Mg2+-ATPase activities in normal, regulating and malignant cells.BiochimicaetBiophysicaActa,684. 67-74.
- 67.U K Laemmli. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature. 227-680.
- 68.Chomcynski P, Sacchi N. (1978) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.Analytical. , Biochemistry 162, 156-159.
- 69.Manikandan R, Thiagarajan R, Beulaja S, Sudhandiran G, Arumugam M. (2010) Curcumin prevents free radical-mediated cataractogenesis through modulations in lens calcium.Free. , Radical Biology Medicine 48, 483-492.
- 70.Yao K, Rao H, Wu R, Tang X, Xu W. (2006) Expression of Hsp70 and Hsp27 in lens epithelial cell in contused eye of rat modulated by thermotolerance or quercetin.Molecular Vision. 12, 445-450.
- 71.J D Rhodes, Sanderson J. (2009) The mechanisms of calcium homeostasis and signalling in the lens.Experimental. , Eye Research 88, 226-234.
- 73.Páramoa B, Montiela T, D R Hernández-Espinosab, Rivera-Martíneza M, Moránb J et al. (2013) Calpain activation induced by glucose deprivation is mediated by oxidative stress and contributes to neuronal damage.TheInternational. , Journalof Biochemistry & Cell Biology 45, 2596-2604.
- 74.Bhandary B, Marahatta A, H R Kim, H J Chae. (2013) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases.International. , Journal of Molecular Science,14 434-456.
- 75.Radi R. (2013) Peroxynitrite, a stealthy biological. , oxidant.Journal of Biological Chemistry 288, 26464-26472.
- 76.Malhotra A, Bath S, Elbarbry F. (2009) An organ system approach to explore the antioxidative, anti-inflammatory, and cytoprotective actions of resveratrol.Oxidative Medicine Cellular Longevity.2015(803971).
Cited by (1)
- 1.Liu Kangjing, Xing Shanghua, Abd El-Aty A.M., Tan Mingqian, 2025, Precision nutrition based on food bioactive components assisted by delivery nanocarriers for ocular health, Trends in Food Science & Technology, 157(), 104923, 10.1016/j.tifs.2025.104923
