Author Contributions
Academic Editor: Pavel Chernyshov, National Medical University, Department of Dermatology and Venereology. Kiev, Ukraine.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2015 N. Haering, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Introduction
Basal cell carcinoma (BCC) is among the most common cancers in Europe, North America and Australia with an estimated 2.8 million cases in the US each year 1, 2. According to the European Skin Cancer Foundation the incidence for BBC in Europe is about 50 to 80 new patients per 100.00 persons per year and in Australia about 250 per 100.00 persons per year 3. The gold standard of treatment for BCC is Mohs micrographic surgery 4. However, surgery is not always possible due to large tumours, problematic locations (e.g. the face), multimorbid patients or the decision of the patient. For this patient cohort multiple different topical therapies such as the photodynamic therapy (PDT) have been established 5. With regard to topical 5% Imiquimod (IQM, Aldara®, Meda Pharma S.A./N.V., Brussels, Belgium) the approved dosage for superficial BCC is 5x/week for altogether 6 weeks. In Europe, 34% of men and 18% of women older than 70 years of age develop actinic keratosis (AK), also known as solar keratosis or keratinocytic intra-epidermal neoplasia 6. Although the probability that AK transforms into squamous cell carcinoma is quite low, the course is unpredictable which makes the therapy of AK a necessity. While single lesions are usually treated with cryosurgery, multiple lesions associated with the phenomenon of field cancerisation are asking for a more widespread topical approach 7. The IQM regimen is defined as applications 3x/week for 4 weeks followed by a 4 week therapy free interval. Eight weeks after initiation of the therapy, the physician is asked to decide on the necessity of a second 3x/week 4 week treatment course 8, 9, 10, 11.
IQM acts as a Toll-like receptor agonist (TLR-7 and TLR-8), thus inducing the production of pro-inflammatory cytokines and subsequent cytotoxic T-cell mediated cell death, but also TLR-independent pathways for apoptosis and inflammation have been reported 12. On clinical grounds, the IQM–related therapeutic effects individually differ in their strength by forming just skin erythema and crusting up to erosions and ulcerations. An essential weakness of the IQM therapy in BCC and AK is the danger of inadequate or missed application by the patient and long therapy periods aggravated by the burden of the IQM induced skin reactions.
The P.L.E.A.S.E. laser system (Pantec Biosolutions, Ruggell, Liechtenstein) is a fractionated erbium: YAG laser with the potency to set per shot one field of up to several hundred micropores into the epidermis down to the dermo-epidermal junction 13.
The novel diode pumped Er:YAG laser P.L.E.A.S.E. Professional (Pantec Biosolutions, Ruggell, Liechtenstein) is fundamentally different to other commercial lamp pumped Er:YAG lasers from the literature. It is capable of creating very precise and homogeneous micropores and the ablation depth can we finetuned in small steps13. In addition, the average power directed on a designated skin area is much higher than lamp pumped Er:YAG lasers.
Multiply, it has been shown that the controlled microporation (SMART PORATION™) is able to increase the transdermal delivery of different drugs 13, 14, 15, 16, 17. This phenomenon together with the much higher application power than conventional lamp pumped Er:YAG lasers turned out to also facilitate immunological reactions such as vaccination and suppressive immunotherapy for allergic asthma in mice 14, 15. Consequently, we hypothesized that this new laser system in combination with topical imiquimod might be able to overcome imiquimod weaknesses by generating shorter clearence times in AK and BCC The two pilot studies herein presented, which have nothing in common with the therapeutic use of conventional lamp pumped Er:YAG lasers in AK and BCC, mainly focused on dose finding aspects such as the frequency of application, laser shots and pulses needed per application and tolerability as well as safety aspects. In addition clearence rates were also analysed, yet, knowing the limitations due to the all-in-all small patient cohort.
Ethics
Studies were approved by the ethical committee of Vorarlberg, EC-No. 2010-5/1_PLEASE-BAS and EC-No. 2010-5/2_PLEASE-AK. The studies have been registered by the European Clinical Trials Database (EudraCT). EudraCT-No. 2010-022521-15 (BCC study) and EudraCT-No. 2010-022502-40 (AK study).
Materials and Methods
Patients and Lesions
The study was performed at the out-patient clinic of the Dept. of Dermatology and Venerology, Federal Academic Teaching Hospital Feldkirch. All patients gave written consent before entering the studies. Exclusion criteria comprised persons under the age of 18 and over 85 years, those who were pregnant or breastfeeding, patients with a personal or familial tendency to develop keloids, and patients intolerant to IQM. AK and BCC located in the hair of the caput, at the genitals less than 5 cm from the eyes and at skin sites which do not allow to use the laser were also excluded. All kinds of histopathologically proven BCC (13 superficial, 5 nodular, infiltrating 2) also comprising recurrent or incompletely excised BCC and AK starting with grade II 18 were included in the studies.
Laser and Topical IQM
Skin microporation was performed using the fractionated erbium:YAG laser device. The laser generated pulses are in the mid infrared spectrum (wavelength 3µm) with an 1mJ energy at 250 Hz, which translates to 0.25 W average power. Typically, 300 pores/cm2 per shot were set. AK and BCC lesions were microporated with 1-3 overlapping shots at a pulse rate of 3-10 (Figure 1a and b). Next IQM was applied to the micropores by the investigator.
Figure 1.Dermoscopic pictures of two microporated and imiquimod treated lesions: a) Arm 4 basal cell carcinoma showing an initial erythema at day 2. Microporation was performed with 1 shot at 10 pulses. b) Arm 2 actinic keratosis showing erythema, crusting and erosions at day 11. Microporation was performed with 2 shots at 3 pulses.
Study Protocol
Both studies represent prospective, monocentric, randomised, case controlled, 4-arm, dose finding clinical pilots. Eligible patients were included by a randomisation schedule conducted sequentially, such as the first patient was allocated to the control arm 1, the second to arm 2, the third to arm 3 and so on 19. In the control arm 1 patients were using topical IQM alone as approved by the regulatory authorities (5x /week for BCC patients; 3x /week for AK patients). In the dose finding arms 2 to 4 the frequency of the combined lased and IQM application was 1x/week, 2x/week and 3x/week, respectively (for the BCC study design see Figure 2). Visits were performed at the study days (Vd) 0, 2, 4, 7, 9, 11, 13, and 20. At the last visit a 3 mm punch biopsy was taken and histopathologically analysed to test clearence of the neoplastic lesions. Consequently, the study period counted for 20 days. Those lesions which did not disappear at V20 were further treated with IQM 3x/week for 4 weeks (AK), or 5x/week for additional 3 weeks (BCC, control arm 1). With regard to BCC in arm 2-4, we fully excised the lesions which did not clear.
Study Parameters
The primary endpoint of the study was defined as the strength of IQM-induced skin reactions (erythema, erosion and crusting) at V20. With the exception of erythema, the quality of which was defined by the Dermaspectrometer® (Cortex Technology, Hadsund, Denmark) 20, all other therapeutic skin reactions were measured with the Global Physician Assessment (PGA) Score ranging from 1 (no reaction) to 4 (very strong reaction) by the investigator and another dermatologist unrelated to the study. The mean value from both examinations was then used as final PGA score. Second study parameters comprised the quality of the therapeutic skin responses at V2, V4, V7, V9, V11 and V13, assessed the same way as at V20. In addition, we asked the patients what they felt during and after the use of the laser (burning, itching, others; V0, V2, V4, V7, V9, V11, V13 and V20), and looked for severe adverse or adverse events at every visit. At V20, a skin punch biopsy was taken from the treated skin, hematoxilin/eosin stained and analysed by a pathologist for the potential disappearance of the neoplastic proliferations.
Results
BCC Study
All in all, 16 patients (4/control arm 1, 3/arm 2, 4/arm 3, 5/arm 4) and 20 lesions (6/control arm 1, 3/arm 2, 5/arm 3, 6/arm 4) were treated. From these 13 patients (3/control arm 1, 3/arm 2, 3/arm 3, 4/arm 4; 8males/5 females; age range 51-81years) and 15 lesions (4/control arm 1, 3/arm 2, 4/arm 3, 4/arm 4) fully adhered to the study protocol receiving therapy throughout the whole study period. In 2 patients (1/arm 3+4) the fractionated erbium:YAG laser and IQM application was stopped at V11 due to a strong skin reaction, but by performing the final punch biopsy at V20 the clearence result of these lesions were counted into the study results. One patient pre-terminated the study due to his will and was considered a drop-out.
In contrast to IQM alone, the combined use of the fractionated erbium:YAG laser microporation with IQM dominantly induced stronger skin erythema levels starting from visit V2 throughout the last visit V20 (Figure 3a). For the stronger therapeutic responses of crusting and erosions this phenomenon was even more pronounced. By looking at the mean crusting PGA values of V2, V4 and V7 together as early time point of the therapy, arm 2-4 presented with the scores of 0.76±0.3, 1.25±0.2 and 1.3±0.5 compared to the much less pronounced value of crusting in the single IQM control arm-1 with 0.3±0.2. The same is true for the late therapy time point V9/11/13/20 with crusting 2.25±0.8, 2.5±0.5 and 2.3±0.4 arms 2-4 and only 1.3±0.6 in the control arm. For erosions the following mean PGA scores were delineated: V2/4/7 arm 1-4 0.08±0.1, 0.6±0.3, 0.75±0.2, 0.95±0.5 and V9/11/13/20 arm 1-4: 0.5±0.25, 1.2±0.6, 1.37±0.3, 1.44±0.4. Regarding the IQM skin responses no correlation could be found with the amount of shots and pulses used by the laser device. Histopathology of V20 lesional punch biopsies showed in all BCC a dense infiltrate of inflammatory cells which is typical for an adequate IQM response. When this infiltrate was fully replacing signs of BCC the lesions were defined as fully cleared (Figure 4). Within arm 2-4 BCC lesions cleared at 33% (persistent 1 nodular and 1 superficial BCC), 100% and 100% at V20, respectively. Taken together IQM alone led to clearence of 3 out of 4 (75%, persistent a superficial) BCC, though the treatment period was shortened from 6 to 3 weeks. The combined fractionated erbium:YAG laser and IQM application resulted in a slightly better clearance rate of 14 BCC vs. 4 (78%). Notably, those 2 BCC patients, where the laser and IQM application was stopped at V11 also showed full clearence. Figure 5 presents the typical treatment course of a patient in arm 3. Tolerability of the laser microporation was excellent with slight burning or the feeling of small needle sticks during the application. In addition, microporation did not cause any immediate irritation of the skin. Even when erythematous, crusted or eroded IQM lesions were microporated the good tolerability stayed the same. No severe adverse or adverse reactions were noted.
Figure 3.Course of skin erythema:a) Basal cell carcinoma study.b) Actinic keratosis study.
Figure 4.Histopathology of a laser and imiquimod treated basal cell carcinoma at the end of the study (day 20).Focal epidermal necrosis with moderate granulocytic demarcation and reactive perivascular lymphocytic and eosinophilic infiltrates. No residual neoplastic proliferates (HE, x200).
Figure 5.Sequence of the typical therapeutic skin reactions in an arm 3 basal cell carcinoma patient at all visits: Days 0, 2, 4, 7, 9, 11, 13, 20 (a-h)
AK Study
We enrolled and treated 18 patients (7/control arm 1, 4/arm 2, 4/arm 3, 3/arm 4), or 21 defined AK lesions (7/control arm 1, 5/arm 2, 5/arm 3, 4/arm 4). Five patients dropped out of the study due to protocol violation (3 patients arm 1, 2, 3) and pre-termination on the basis of adverse IQM reactions with strong erythema, ulceration and systemic flue like symptoms (2 patients control arm 1). Consequently 13 patients (4/control arm 1, 3/arm 2, 3/arm 3, 3/arm 4) or 15 lesions were (4/control arm 1, 4/arm 2, 3/arm 3, 4/arm 4) analysed and counted.
As seen in the treatment of BCC, AK microporation with IQM also led to essentially the same stronger skin erythema (with one unique peak at V13 in the control arm 1) compared to IQM alone (. Figure 3b). In addition, mean PGA scores for crusting and erosion of control arm 1 in relation to arms 1-4 were just lower in the beginning of the therapy (mean PGA at V2/4/7-arm 1-4 for crusting: 0.42±0.3, 0.83±0.3, 1.12±0.1, 1.16±0.1; erosion: 0±0, 0.3±0.2, 0.43±0.2, 0.41±0.1) and reached the same levels towards the end (mean PGA at V9/11/13/20-arm 1-4 crusting: 1.5±0.3, 1.5±0.2, 2.15±0.1,1.7±0.3; erosion: 0.7±0.1, 0.6±0, 1.2±0.1, 0.71±0). In arm 2 50% of AK lesions, in arm 3 100% and in arm 4 75% cleared. Taken together, the clearence rate in the fractionated erbium:YAG laser plus IQM arms counted for 75% or 10 AK vs. 50% or 2 AK in the control arm. One patient from arm 2 was followed up to 6 months and showed no relapse of AK (Figure 6). Tolerability of the laser microporation device was the same as seen with BCC treatment and no sever adverse reactions were noted.
Figure 6.Long-term follow up of an actinic keratosis patient. a) Initial situation, b) day 20 at the end of the study, c) 6 month later. Notably, no relapse.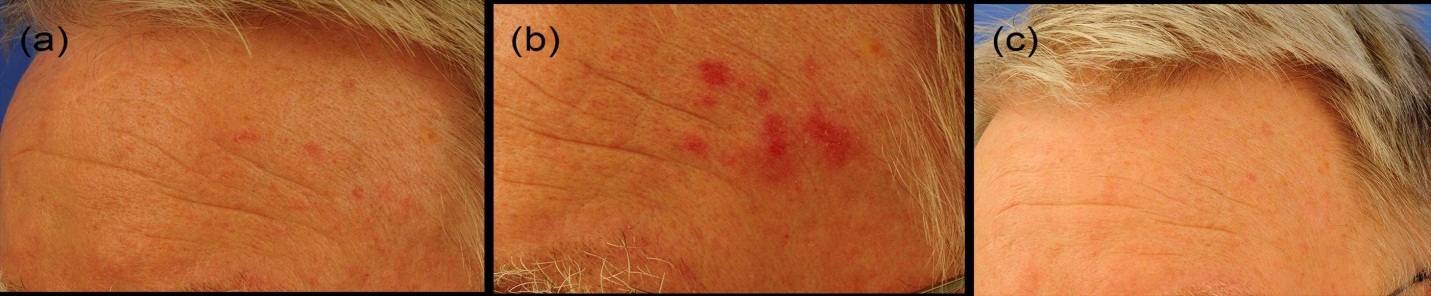
Discussion
Both, IQM and PDT are well established therapies for BCC and (multiple) AK. Nevertheless, they also harbour some shortcomings and inconveniences. While PDT (i) needs a long waiting time for the patient, (ii) is often painful with (iii) frequent needs of multiple therapeutic cycles, IQM represents a therapy of long duration (AK up to 12 weeks, BCC 6 weeks) which might be associated with problematic compliance, or wrong use by the patient. We herein report a new treatment option for BCC and AK consisting of a fractionated erbium:YAG laser induced skin microporation followed by an over the micropores application of IQM which has the potency to overcome IQM and PDT problems. In this context it is important to emphasize that the laser system used is fundamentally different to other commercial lamp pumped Er:YAG lasers reported in the literature for the direct treatment of AK and BCC. In our studies IQM is applied immediately after the microporation by the care giver and not the patient. Second, our study showed that the therapy is associated with minimal adverse perceptions (mostly like small needle sticks) and needs shorter treatment times as well as lower overall IQM exposition than IQM alone. In detail, our new therapy was performed for only 20 days, while the approved IQM use is up to 12 weeks (AK) and with regard to BCC 6 weeks. In addition, the IQM exposition represented an application 1x/week (arm 2), 2x/week (arm 3) and 3x/week (arm 4) compared to 3x/week (AK) and 5x/week (BCC), respectively. Although we are aware of the small sample size, it must be noted that, despite the specifics of this new therapy, the overall clearence rate of BCC was comparable to IQM alone and, in the case of AK, even better.
IQM effects on microporated skin, i.e. the nature and quality of the therapeutic skin reactions erythema, crusting and erosions are essential to understand the efficacy of this new therapeutic approach. Erythema representing the most solid IQM reaction appeared already after day 2 and initially was stronger compared to erythema in skin treated with IQM alone. Notwithstanding this early strong reaction, at the later phase of the therapy (V11/13/20) levels of skin erythema in microporated and non-microporated skin were essentially the same. By looking at the more pronounced IQM skin reactions such as crusting and erosions, in AK their behaviour was comparable to erythema, while in BCC their late phase strength was also stronger than IQM alone. Based on the IQM mode of action, it seems obvious that the differences in the therapeutic skin reactions might be caused by the application of IQM via the micropores which leads to a faster and closer IQM contact with the epidermal dendritic cells. Based on this mechanism, we would suggest to call the combined therapeutic use of the fractionated erbium:YAG laser microporation with IQM “laser dynamic therapy (LDT)”.
In order to identify the most appropriate laser and IQM frequency, which was the major goal of our pilot studies, we first had to correlate the number of applications per week with safety and tolerability aspects. However, this correlation was not helpful, since the only 2 adverse reactions seen were found in the control arm 1 (IQM alone) of the AK trial. We then looked at the efficacy parameters to solve that question and found out that in both studies arm 3 was associated with a clearence rate of 100%. Regarding the laser shots per application and the different pulses used no correlation with safety and efficacy aspects could be found. Taken together, by a synergistic view of our data, we would like to recommend the laser microporation with 2-3 shots (depending on the size of the lesion) at a pulse number of 3 two times per week together with the topical use of IQM for altogether 20 days. At this point, the good safety and efficacy ratio seemingly makes LDT a valid new therapeutic option for AK and BCC patients. It goes without saying that our findings have to be substantiated with further studies of a larger patient cohort.
Acknowledgments
The authors thank Dr. Schuff for assisting as medical writer in the preparation of the first draft manuscript.
Conflicts of Interest:
Dr. Strohal reports serving on speakers bureaus for Pfizer, Schülke and Mayer, Lohmann und Rauscher, Meda Pharmaceuticals, Menarini Pharmaceuticals, Stockhausen, and Smith + Nephew; having consulting agreements with Pantec, Pfizer, Astellas, Novartis, Lohmann und Rauscher, Urgo, Chemomedica, Schülke and Mayer and Pantec Biotechnologies; receiving research and educational grants from Pantec, Stockhausen, 3M-Woundcare, Smith + Nephew, Lohmann und Rauscher, Enjo Commercials, Urgo, Chemomedica and Schülke and Mayer. Dr. Häring and Dr. Barlas report no conflict of interest. Dr. Boehler is the CEO of Pantec Biosolutions.
Funding Sources:
The study was sponsored by Pantec Biosolutions. Financial medical writing support was provided by Pantec Biosolutions.
References
- 1.Smith V, Walton S. (2011) Treatment of facial Basal cell carcinoma: a review. , J Skin Cancer2011: 380371.
- 2.Skin Cancer Foundation Skin cancer facts. Available at: www.skincancer.org/skin-cancer-information/skin-cancer-facts. Accessed February27,2012.
- 3.European Skin Cancer Foundation. Available at:. http://www.escf-network.eu/en/patients/skin-cancer/basal-cell-carcinoma.html. Accessed November5,2013
- 4.Rowe D E. (1995) Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 13, 617-20.
- 5.Matei C, Tampa M, Poteca T. (2013) Photodynamic therapy in the treatment of basal cell carcinoma. , J Med Life 6, 50-4.
- 6.Hensen P, Müller M L, Haschemi R. (2009) Predisposing factors of actinic keratosis in a North-West German population. , Eur J Dermatol 19, 345-54.
- 7.Stockfleth E. (2012) The paradigm shift in treating actinic keratosis: a comprehensive strategy. , J Drugs Dermatol 11, 1462-7.
- 8.Alomar A, Bichel J, McRae S. (2007) Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. , Brit J Dermatol 157, 133-141.
- 9.Jorizzo J, Dinehart S, Matheson R. (2007) Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week on one or two courses of treatment of actinic keratoses on the head. , J Am Acad Dermatol 57, 1-4.
- 10.Salasche S J, Levine N, Morrison L. (2002) Cycle therapy of actinic keratoses of the face and scalp with 5% topical imiquimod cream: An open-label trial. J Am Acad Dermatol. 47, 1-9.
- 11.Stockfleth E, Sterry W, Carey-Yard M. (2007) Multicentre, open-label study using Imiquimod 5% cream in one or two 4-week courses of treatment for multiple actinic keratoses on the head. , Brit J Dermatol 157, 41-46.
- 12.Walter A, Schäfer M, Cecconi V. (2013) Aldara activates TLR7-independent immune defence. , Nat Commun 4, 1560.
- 13.Bachhav Y G, Heinrich A, Kalia Y N. (2011) Using laser microporation to improve transdermal delivery of diclofenac: Increasing bioavailability and the range of therapeutic applications. , Eur J Pharm Biopharm 78, 408-14.
- 14.Bach D, Weiss R, Hessenberger M. (2012) Transcutaneous immunotherapy via laser-generated micropores efficiently alleviates allergic asthma in Phl p 5-sensitized mice. Allergy. 67, 1365-74.
- 15.Weiss R, Hessenberger M, Kitzmüller S. (2012) Transcutaneous vaccination via laser microporation. , J Control Release 162, 391-9.
- 16.Yu J, Bachhav Y G, Summer S. (2010) Using controlled laser-microporation to increase transdermal delivery of prednisone. , J Control Release 148, 71-3.
- 18.Carducci M, Bozzetti M, Foscolo A M. (2011) Margin detection using digital dermatoscopy improves the performance of traditional surgical excision of basal cell carcinomas of the head and neck. Dermatol Surg. 37, 280-5.
Cited by (5)
- 1.Fredman Gabriella, Wenande Emily, Hendel Kristoffer, Togsverd‐Bo Katrine, Haedersdal Merete, 2022, Efficacy and safety of laser‐assisted combination chemotherapy: A follow‐up study of treatment with 5‐fluorouracil and cisplatin for basal cell carcinoma, Lasers in Surgery and Medicine, 54(1), 113, 10.1002/lsm.23497
- 2.Zech Herbert, Murtinger Maximilian, 2020, , , (), 103, 10.1007/978-981-15-2377-9_12
- 3.Erlendsson Andrés M., Olesen Uffe H., Haedersdal Merete, Rossi Anthony M., 2020, Ablative fractional laser-assisted treatments for keratinocyte carcinomas and its precursors–Clinical review and future perspectives, Advanced Drug Delivery Reviews, 153(), 185, 10.1016/j.addr.2020.01.001
- 4.Wenande Emily, Hendel Kristoffer, Mogensen Mette, Bagger Charlotte, Mårtensson Nina L., et al, 2021, Efficacy and Safety of Laser‐Assisted Combination Chemotherapy: An Explorative Imaging‐Guided Treatment With 5‐Fluorouracil and Cisplatin for Basal Cell Carcinoma, Lasers in Surgery and Medicine, 53(1), 119, 10.1002/lsm.23323
- 5.Scheiblhofer Sandra, Strobl Anna, Hoepflinger Veronika, Thalhamer Theresa, Steiner Martin, et al, 2017, Skin vaccination via fractional infrared laser ablation - Optimization of laser-parameters and adjuvantation, Vaccine, 35(14), 1802, 10.1016/j.vaccine.2016.11.105

