Pancreatic cancer in a Non-Tertiary Center: CEA and Bilirubin More Predictive than CA 19-9 at Initial Presentation
Abstract
Background
This study was undertaken to delineate the most predictive pattern at presentation for patients with non-metastatic pancreatic cancer compared to patients with metastatic pancreatic cancer who present to a medium sized hospital.
Methods
Data were collected at a medium sized hospital from 2009-2014 for patients with newly diagnosed pancreatic adenocarcinoma. Laboratory values, CT scans, pathology reports and ERCP results were obtained. Data are presented as mean (median +/- standard deviation).
Results
Fifty-two patients met the criteria for inclusion and were clinically diagnosed with pancreatic cancer. The median age was 71 years old. CEA levels for metastatic pancreatic cancer were 107.9 (20.6 +/- 166.7) ng/dl and 9 (6 +/- 11.6) ng/dl for non-metastatic cancer (P-value<0.05).
Bilirubin levels for metastatic pancreatic cancer were 4.1 (0.7 +/- 6.6) mg/dl and 10.3 (10.4 +/- 8.1) mg/dl for non-metastatic cancer (p=0.009). CA19-9 levels for metastatic pancreatic cancer were 37,529 (644 +/- 88,352) U/ml and 5,150 (668 +/- 16,985) U/ml for non-metastatic cancer.
Conclusion
Elevated total bilirubin alongside low CEA appears to be a stronger predictor of non-metastatic disease at presentation compared to CA 19-9 alone.
Author Contributions
Academic Editor: Ian James Martins, Edith Cowan University.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2025 Majd Al Masri, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Pancreatic cancer is one of the deadliest and most aggressive cancers and is currently the third leading cause of cancer related deaths in the United States, surpassing breast cancer, and even expected to overtake colon cancer as the second place1, 2. The overall 5-year survival rate for pancreatic cancer is 9%3, 4, 5 and 3% for metastatic pancreatic cancer. The most common pancreatic cancer subtype is pancreatic adenocarcinoma6, 7, 8, 9, while the second most common are pancreatic neuroendocrine neoplasms2.
Even though the prognosis and survival rate of pancreatic cancer seem so dismal10, 11, studies have shown that the survival for non-metastatic early stage pancreatic adenocarcinoma is relatively high. Pancreatic adenocarcinoma in situ (stage 0) has a 5-year survival rate of 85% and 69% for stage 1A12, which makes early detection and diagnosis the most important, yet challenging, step of managing pancreatic cancer12, 13. Surgical resection continues to be the only hope for cure for pancreatic cancer2, 14, 15.
The only serum biomarker that is approved by the United States Food and Drug Administration for routine management of pancreatic cancer is Cancer Antigen 19-9 (CA 19-9), but due to it’s low positive predictive value, it is only appropriate for detecting cancer recurrence and response to treatment16, 17. Cancer embryonic antigen (CEA) seems to be a more clinically significant biomarker in prediction of advanced pancreatic cancer when compared with CA 19-918. We undertook this study to examine the usefulness and practicality of CEA and bilirubin levels as a predictor of metastatic state for patients with pancreatic cancer presenting to a non-tertiary center.
Methods
We retrospectively reviewed the charts of patients who were newly diagnosed with pancreatic cancer and were admitted from the emergency department from 2009-2014 at a medium-sized non-tertiary hospital. Pertinent labs, imaging, pathology results, dates of diagnosis, consultations ordered, procedures, operations, and adjuvant therapy data were
recorded and analyzed. Categorical variables were compared using Chi-squared or Fisher’s exact tests, as appropriate. Kaplan-Meier survival curves were generated to visualize survival differences, and statistical significance between groups was assessed using the log-rank test.
Continuous variables are presented as medians, and they were compared using the Mann-Whitney U test or analysis of variance. Data are reported as median (mean +/- standard deviation). Patients included in this study presented to the emergency department at the medium-sized hospital and were newly diagnosed with pancreatic cancer during the 5-year period.
Results
Fifty-two patients met the criteria for inclusion and were clinically diagnosed with pancreatic cancer. The majority were men (56%). The median age was 71 years old with the ages ranging from 55 to 90 years old. Thirty-three patients diagnosed with pancreatic cancer were confirmed by biopsy. The pathologic subtypes were adenocarcinoma (28), neuroendocrine (3), squamous cell (1) and cholangiocarcinoma (1) (Figure 1). Most of the patients had the cancer localized (50), with the majority localized in the pancreatic head (28), while the other 25 were located in the pancreatic tail (10), pancreatic body (11) or classified as an overlapping lesion of pancreas (Figure 2). Twenty-seven patients were diagnosed with clinical metastatic disease and 23 patients were diagnosed with non-metastatic disease.
Figure 1.Number of pancreatic cancer cases based on pathologic subtypes (n=33)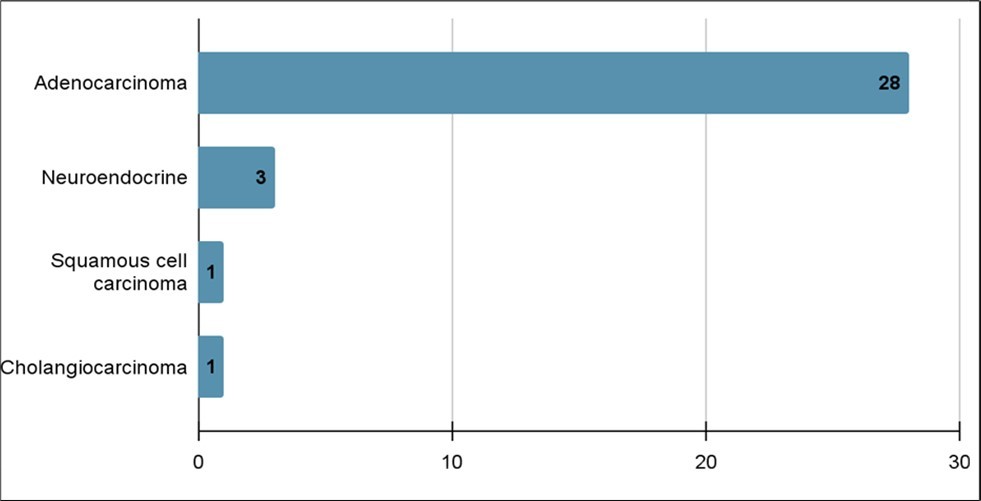
Figure 2.Number of pancreatic cancer cases based on location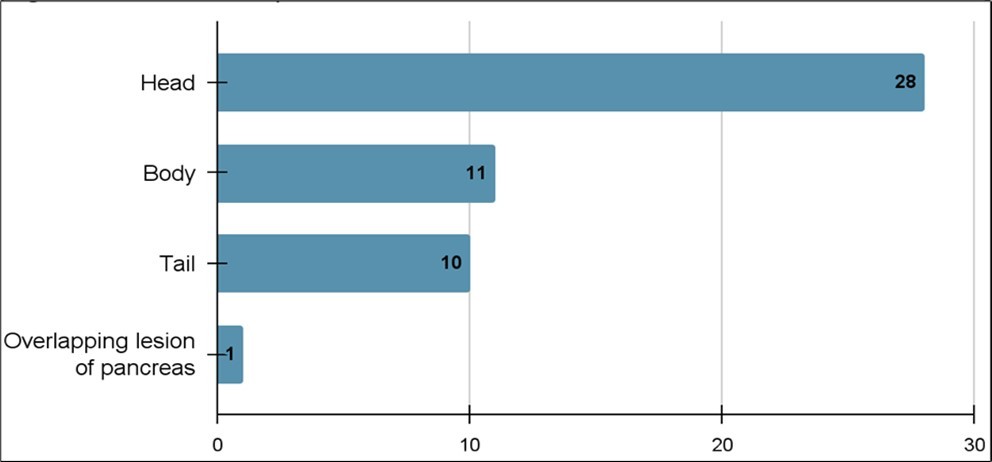
The initial workup of diagnosing pancreatic cancer includes ordering multiple laboratory tests, most notably bilirubin, CEA and CA19-9. Thirty patients had their serum CEA tested, the mean (median +/- standard deviation) CEA level for metastatic pancreatic cancer was 107.9 (20.6 +/- 166.7) ng/dl and 9 (6 +/- 11.6) ng/dl for non-metastatic cancer (Figure 3), and of the patients that had a CEA value > 8, 10 had metastasis at the time of presentation, compared to three patients without metastatic disease. Four patients with metastases had a CEA < 8 compared to 10 without metastases (p=0.02). Forty-six patients underwent bilirubin testing and the mean (median +/- standard deviation) bilirubin for those diagnosed with metastatic cancer was 4.1 (0.7 +/- 6.6) mg/dl, while the mean (median +/- standard deviation) bilirubin of those diagnosed with non-metastatic cancer was 10.3 (10.4 +/- 8.1) mg/dl (p=0.009) (Figure 4). CA19-9 testing was undertaken for 42 patients. The mean (median +/- standard deviation) CA19-9 level for metastatic pancreatic cancer was 37,529 (644 +/- 88,352) U/ml and 5,150 (668 +/- 16,985) U/ml for non-metastatic cancer. Seven patients with metastatic disease had a CA19-9 > 5000 compared to two patients without metastasis. Eight patients with metastatic disease presented with a CA 19-9 < 5000 compared to 15 patients without metastases (p= 0.49).
Figure 3.Average CEA in metastatic vs non-metastatic Pancreatic cancer. (p-value<0.05)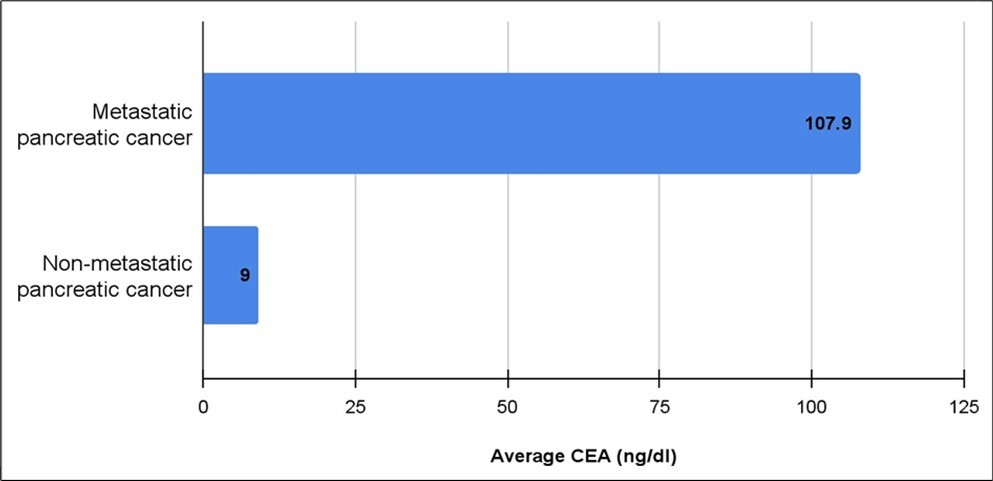
Figure 4.Average total bilirubin for patients with metastatic vs. non-metastatic pancreatic cancer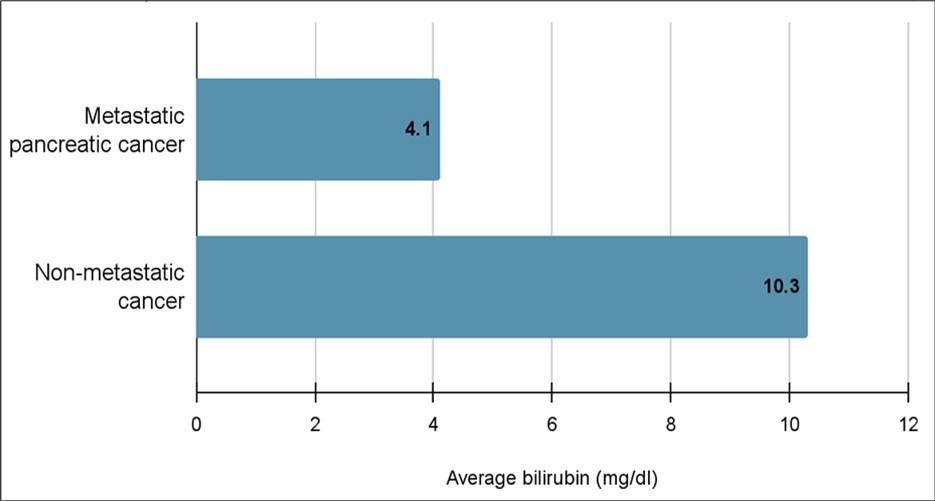
Twenty-one patients had ERCPs undertaken and 14 of those patients had stent placements. Five patients underwent a pancreaticoduodenectomy, two patients underwent a distal pancreatectomy with splenectomy, six patients underwent laparoscopies and one patient underwent an extended distal pancreatectomy. The consult frequencies were as follows: gastroenterology (66%), oncology (61%), and general surgery (39%) (Figure 5). The time from diagnosis to port placement was 22.5 days and time from diagnosis to surgery was 10 days.
Figure 5.Percentage of consults requested during pancreatic cancer management at a non-tertiary hospital.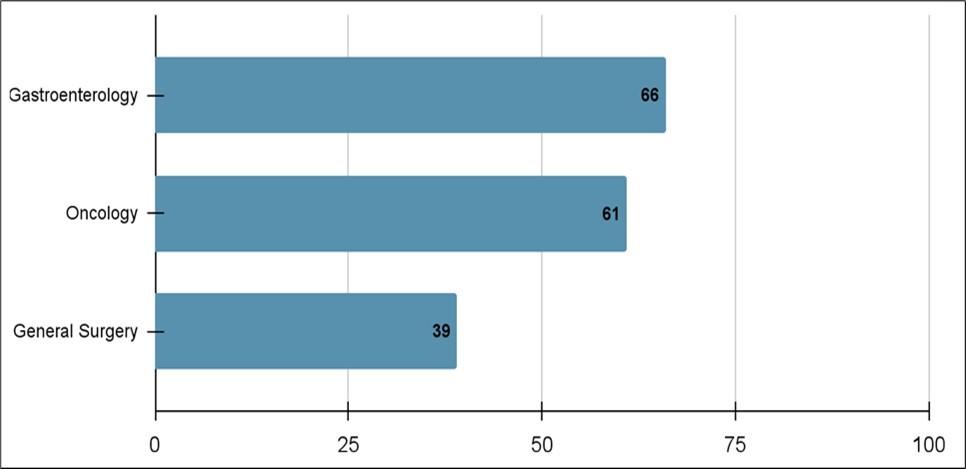
The survival between patients with metastatic vs non-metastatic disease was statistically significant (p= 0.01) (Figure 6). Moreover, there was a statistically significant difference in survival between patients with normal bilirubin and CEA vs abnormal bilirubin and CEA (p=0.02) (Figure 7) (Figure 8) (Figure 9). The comparison between normal vs abnormal bilirubin alone and normal vs abnormal CEA alone showed no significant difference (p=0.21, p=0.82 respectively).
Figure 6.Kaplan Meier curve comparing survival between patients with metastatic vs non-metastatic disease.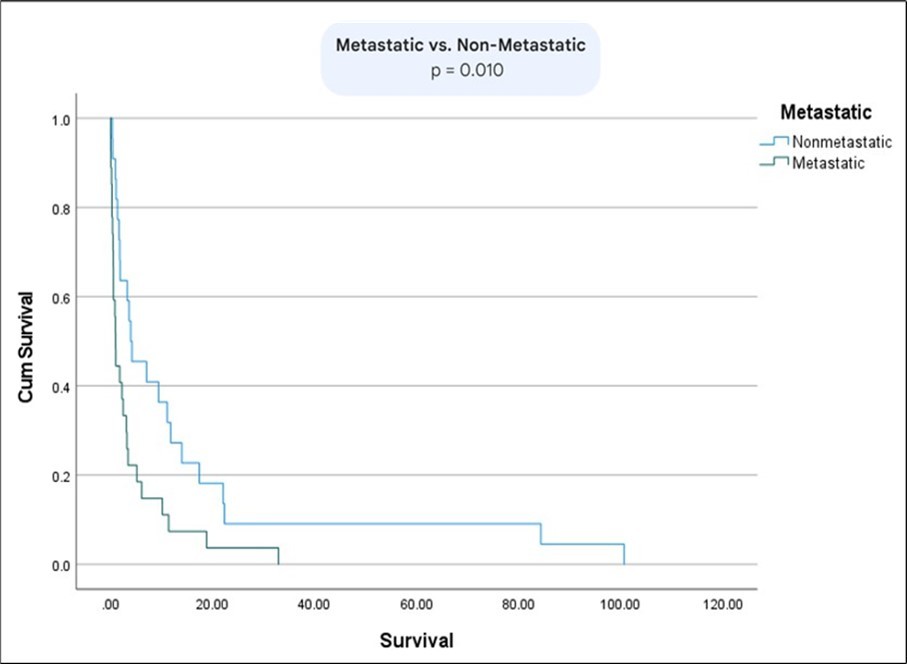
Figure 7.Kaplan Meier comparing survival rates between patients normal CEA vs abnormal CEA.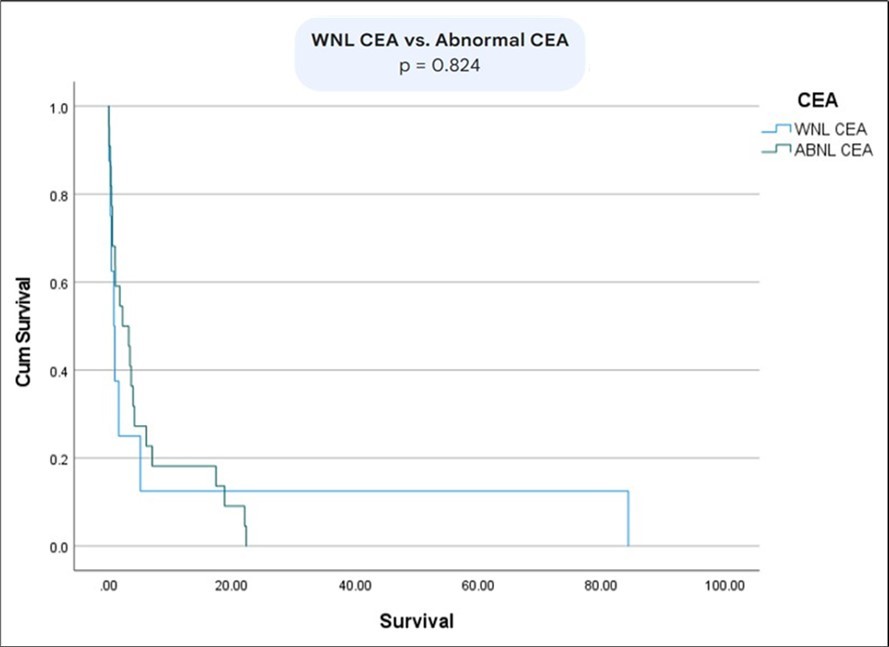
Figure 8.Kaplan meier curves comparing survival between patients with normal bilirubin vs abnormal bilirubin.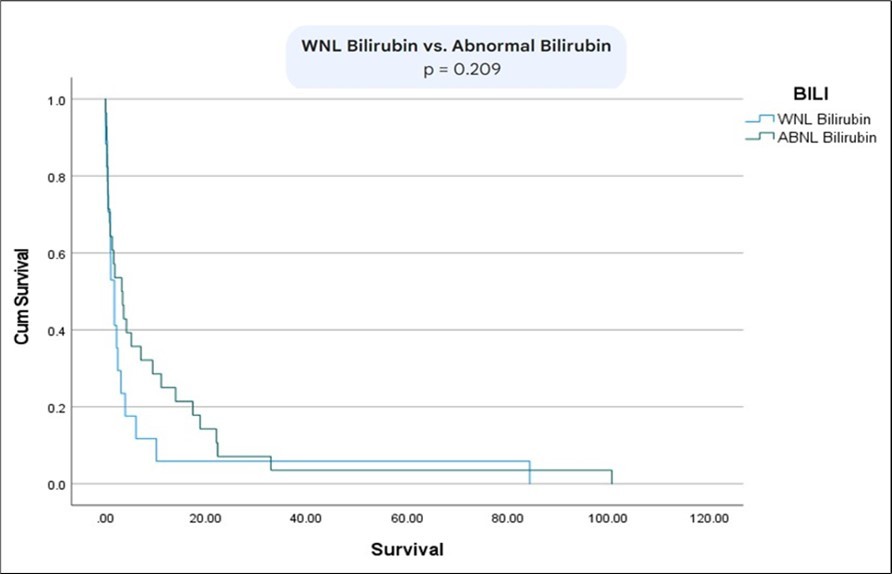
Figure 9.Kaplan Meier curve comparing survival between patients with normal CEA and bilirubin vs abnormal CEA and bilirubin.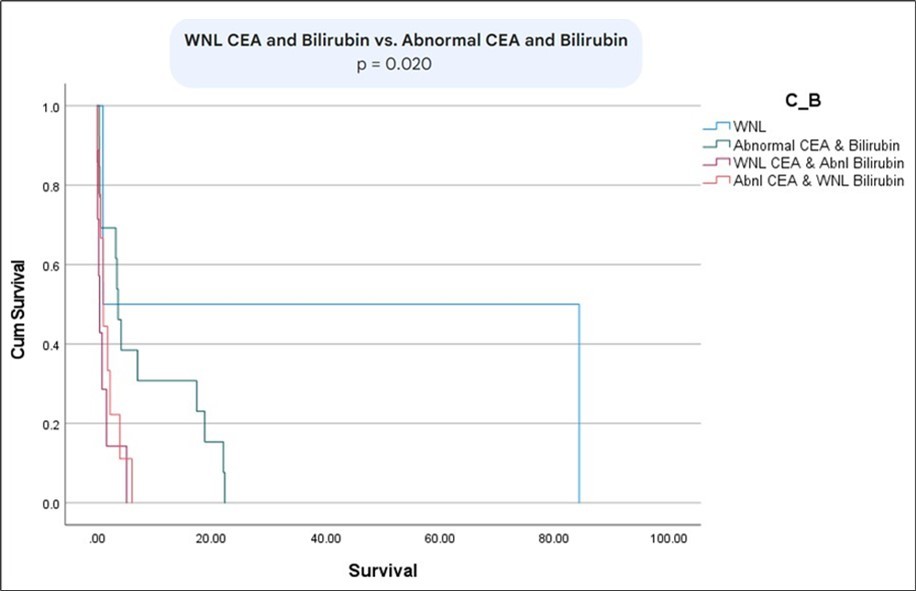
Discussion
Pancreatic cancer remains one of the most challenging and pressing medical concerns worldwide due to its poor survival rates, grim prognosis, and difficulty in detecting metastasis. It is currently the third leading cause of cancer-related deaths in the United States, with a five-year survival rate of just 9% for non-metastatic cases and only 3% for metastatic pancreatic cancer1, 2, 3, 4, 5. The primary danger lies in its late-stage detection, as early diagnosis significantly improves prognosis and treatment outcomes.
Currently, no reliable screening tool exists for detecting pancreatic cancer in its early stages. The only serum biomarker approved by the U.S. Food and Drug Administration for routine management of the disease is Cancer Antigen 19-9 (CA 19-9). However, due to its low positive predictive value, CA 19-9 is primarily useful for monitoring cancer recurrence and treatment response rather than early detection16, 17. Additionally, its levels can be elevated in cases of biliary obstruction, limiting its utility in identifying metastatic disease, as biliary obstruction and metastasis do not always coincide in pancreatic cancer. Therefore, CA 19-9 should be reserved for patients without biliary obstruction later in their disease course, while carcinoembryonic antigen (CEA) should be used early alongside bilirubin levels.
The primary objective of this study was to assess the predictive value of CEA and bilirubin levels, compared to CA 19-9, in determining cancer progression and the presence of metastasis for patients with pancreatic cancer18. Previous research has shown that CEA is significantly elevated for patients with pancreatic cancer. Our findings confirmed this as CEA levels were markedly elevated for patients with metastatic pancreatic cancer (107.9 (20.6 ± 166.7) ng/dL) compared to those with non-metastatic disease (9 (6 ± 11.6) ng/dL). Additionally, the bilirubin level for patients with metastatic disease was 0.7 (4.1 +/- 6.6) mg/dL, whereas it was 10.4 (10.3+/- 8.1) mg/dL for patients with non-metastatic disease. Notably, CA 19-9 levels in our study did not demonstrate significance for detecting metastatic disease.
It is also worth noting that 5–10% of the general population and up to 25% of some ethnic populations harbor homozygous alterations in the FUT2/3 genes, which causes them to be incapable of producing and/or secreting CA 19-9. Additionally, CA 19-9 levels tend to elevate in diabetics and elderly patients19, which is another reason why we believe CA 19-9 cannot be solely relied on as a biomarker for pancreatic cancer.
Additionally, we observed that general surgery consultations were utilized less frequently (39%) compared to gastroenterology (66%) and oncology (61%) consultations. We advocate for earlier surgical consultation in the management of pancreatic cancer, particularly in patients without metastatic disease. Early involvement of surgery can facilitate timely mediport placement and potentially allow for tumor resection if deemed feasible.
This study has several limitations. First, the sample size is relatively small, and further research with larger patient cohorts is needed to better assess the relationship between CEA, total bilirubin levels, and the metastatic status of pancreatic cancer. Additionally, not all patients in our study had complete laboratory data for total bilirubin, CEA, and CA 19-9, which may have impacted the analysis.
Conclusion
Our study suggests that combining CEA and total bilirubin levels may serve as a valuable prognostic tool in pancreatic cancer patients. Elevated total bilirubin alongside low CEA appears to be a stronger predictor of non-metastatic disease at presentation compared to CA 19-9 alone. Additionally, general surgery consultations were likely underutilized, and earlier involvement could have facilitated timely mediport placement and potential surgical intervention.
Abbreviations
References
- 1.C J Halbrook, C A Lyssiotis, Pasca di Magliano, Maitra M, A. (2023) . Pancreatic cancer: Advances and challenges.Cell 186(8), 1729-1754.
- 2.C Y Zhang, Liu S, Yang M. (2022) Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options.World journal of gastroenterology. 28(48), 6827-6845.
- 3.R S O'Neill, Stoita A. (2021) Biomarkers in the diagnosis of pancreatic cancer: Are we closer to finding the golden ticket?.World journal of gastroenterology. 27(26), 4045-4087.
- 4.Ilic M, Ilic I. (2016) Epidemiology of pancreatic cancer.World journal of gastroenterology. 22(44), 9694-9705.
- 5.Gupta R, Amanam I, Chung V. (2017) Current and future therapies for advanced pancreatic cancer.Journal of surgical oncology. 116(1), 25-34.
- 6.M J Amaral, R C Oliveira, Donato P, J G Tralhão. (2023) Pancreatic Cancer Biomarkers: Oncogenic Mutations, Tissue and Liquid Biopsies, and Radiomics-A Review.Digestive diseases and sciences. 68(7), 2811-2823.
- 7.Goral V. (2015) Pancreatic Cancer: Pathogenesis and Diagnosis.Asian Pacific journal of cancerprevention:APJCP. 16(14), 5619-5624.
- 8. (2021) Advancing on pancreatic cancer.Nature reviews. , Gastroenterology & hepatology 18(7), 447-10.
- 9.C, E P Diamandis, Brand R, Rückert F, Haun R et al. (2013) Pancreatic cancer.Clinical chemistry. 59(1), 41-46.
- 10.Cai J, Chen H, Lu M, Zhang Y, Lu B et al. (2021) Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis.Cancer letters. 520-1.
- 11.Liu S, Cai Y, Changyong E, Sheng J, Zhang X. (2021) . Screening and Validation of Independent Predictors of Poor Survival in Pancreatic Cancer.Pathology oncologyresearch :POR 27, 1609868-10.
- 12.Tonini V, Zanni M. (2022) Early diagnosis of pancreatic cancer: What strategies to avoid a foretold catastrophe.World journal of gastroenterology. 28(31), 4235-4248.
- 13.E M Stoffel, R E Brand, Goggins M. (2023) Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention.Gastroenterology. 164(5), 752-765.
- 14.Vincent A, Herman J, Schulick R, R H Hruban, Goggins M. (2011) Pancreatic cancer.Lancet (London. , England) 378(9791), 607-620.
- 15.Masiak-Segit W, Rawicz-Pruszyński K, Skórzewska M, W P Polkowski. (2018) Surgical treatment of pancreatic cancer.Polskiprzegladchirurgiczny. 90(2), 45-53.
- 16.McGuigan A, Kelly P, R C Turkington, Jones C, H G Coleman et al. (2018) Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes.World journal of gastroenterology. 24(43), 4846-4861.
- 17.Bhat K, Wang F, Ma Q, Li Q, Mallik S et al. (2012) Advances in biomarker research for pancreatic cancer.Current pharmaceutical design. 18(17), 2439-2451.
