Application of Acrylates in Enhanced Oil Recovery
Abstract
Currently, enhanced oil recovery (EOR) acquire increased attention to recover more residual oil trapped after the primary and secondary process in petroleum reservoirs. EOR comprise different technologies involving chemical, thermal, miscible flooding techniques. Chemical flooding by the polymer is a widely implemented method on the academic and industrial scale. In this chapter, the authors discuss polymer flooding using polyacrylates that involve hydrolyzed polyacrylamide (HPAM), hydrophobically associated polyacrylamides (HAPAM), which grafted with different vinyl monomers such as acrylic acid, methyl methacrylates, and 2-acrylamido-2-methylpropane sulfonic acid (AMPS). These polymers increase the viscosity of injected brine solutions, as a result, decrease mobility ratio and enhance sweeping efficiency, so the water act as a piston, which pushes oil in front of it, and consequently increase the recovery factor. The advantages and disadvantages of these polymers as well as comparing different flooding scenarios are reported.
Author Contributions
Academic Editor: Xiaoyong Lu, Department of Chemistry and Biochemistry, Ohio University, Athens, Ohio, 45701 USA.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 A.N. El-hoshoudy, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Polymer flooding is a common EOR technique, in which solutions of a high molecular weight water-soluble polymer is pumped into the reservoir in order to increase the water phase viscosity to obtain favorable mobility and minimize channeling effects, improving sweeping and displacement efficiencies 1. With the world’s attention on enhancing fossil-fuel production to satisfy national promotion and daily energy consumptions. Enhanced oil recovery (EOR) is of considerable academic and technological interest2. Crude oil recovery occurs through three distinct phases, firstly production results from the natural energy of the fluids and the rock decompression3, where it recovers 5 to 15% of the initial reserve. The second stage is known as secondary recovery involving the injection of fluids such as water or gas to boast and maintain the reservoir pressure, so force the oil into the wellbores of producing wells and recovery factor reach 10-20% 4, 5, 6. The last stage is combined under the name of Enhanced Oil Recovery (EOR), which recover between 10 and 35% of additional oil and comprise thermal, miscible and chemical methods. EOR can be defined as any processes that increase oil recovery by reduction of the residual oil saturation (Sor) after primary and secondary production. Virtually, EOR technologies involve the injection of fluids not normally present in the reservoir (e.g. polymers, foams, surfactants) in order to increase oil recovery 7. Tertiary or enhanced oil recovery aims to recover the remaining original oil in place (OOIP) 7 and increase the ultimate oil recovery of the reservoir through one or more sophisticated techniques including chemical, thermal, and miscible flooding8 for recovering up to an additional 40 % of the OOIP. Several kinds of literature discuss the application of polymer flooding in enhanced oil recovery. Dabbous,1977 9 conducted the flooding tests in heterogeneous porous media showed that preinjection of the polymer could result in better flooding efficiency. Platt and James,198110 prepared poly (alpha-alkoxy) acrylamides and claimed improved stability in brine solutions. Similar claims were made by Hunter, 2008 11 and Cao et al, 201512who prepared N-substituted PAM/AA via ethoxylation. Talley, 199313 disclosed terpolymers of acrylamide, acrylonitrile and acrylic acid, the latter optionally alkoxylated with ethylene oxide. McCormick et al, 1982&2014 14, 15) copolymerize sulfonated monomers such as 2-acrylamido-2-methylpropane sulfonic acid (AMPS) with acrylamide monomers. Martin et al, 198316 studied the synthesis of polyacrylamide containing imide rings and concluded that they are less susceptible to alkaline hydrolysis than PAM. Moreover, they studied the viscosity retention in brine for hydrolyzed PAM/AA versus copolymerized PAM/AA and found little difference in the two, claiming that shear resistance of the hydrolyzed PAM/AA was superior to the copolymers. Osterloh and Jante, 199217 mention that surfactant adsorption is minimized in the presence of polymer thus the use of a surfactant-polymer flood could be highly favorable and justified. Farouq-Ali and Thomas, 200118 also discuss the limitations of the use of polymer floods and mention that being introduced earlier in the life of a water flood is a better option. They also report incremental oil recoveries in the order of 5% on average. Schramm, 2000 19 discusses the concept of "Low Tension Polymer Flood" (LTPF). He concluded that; the flood in the first instance was conducted by co-injection of the surfactant and polymer and, due to chromatographic effects, the polymer moved ahead of the surfactant. Du and Guan, 200420 discuss the field application of polymer floods and conclude that when applied after the reservoir has been extensively flooded by other means, polymer floods have been unsuccessful. Another observation they make is that in reservoirs of low average permeability, injectivity of the floods greatly reduced with the addition of a polymer and resulted in poor performance. El-hoshoudy et al group 21, 22, 23, 24, 25, 26, 27, 28, 29 discuss the preparation and application of different hydrophobically modified polyacrylates and their modified nanocomposites as well as biopolymers and their composites in the field of enhanced oil recovery. They also reported about the use of 1-vinyl imidazole surfmer for the synthesis of polyacrylamide-SiO2 nanocomposite for severe polymer flooding conditions. Other literature reported about the use of quaternary ammonium-based surfmer with polyacrylamide co-polymer for flooding in carbonate rock30. Others reported about modification of starch biopolymers through grafting with different vinyl monomers to be applied in enhanced oil recovery applications31, 32. Gou et al 33reported about copolymerization of acrylamide (AM), acrylic acid (AA), 1‐acrylamido ethyl‐2‐oleic imidazoline (ACEIM) with the sodium salts of 3‐(diallyl‐amino)‐2‐hydroxypropyl (NDS) or 2‐acrylamido‐2 methylpropane sulfonic acid (AMPS) for polymer flooding at harsh reservoir conditions of high salinity and temperature. Hsieh et al 34 describe the synthesis of water-soluble polymers suitable for enhanced oil recovery applications through polymerization of acrylamide, N-vinyl-2-pyrrolidone, sodium 2-acrylamido 2-methylpropanesulfonate and N, N-dimethylacrylamide. Lai et al 35 synthesize a novel hyperbranched polymer using acrylamide (AM), acrylic acid (AA), N-vinyl-2-pyrrolidone (NVP), and dendrite functional monomer as raw materials by redox initiation system in an aqueous medium to be applied in enhanced oil recovery. Liu et al 36 fabricated a novel star-like hydrophobically associative polyacrylamide used in enhanced oil recovery (EOR) processes in harsh reservoir conditions. Pu et al 2reported about the synthesis of a novel water-soluble core-shell hyperbranched polymers (HBPAMs), consisting of nano silica core, hyperbranched polyamidoamide (PAMAM) as subshell and linear hydrophilic chains as the outermost layer, through in-situ free radical polymerization strategy. Raffa et al 1 fabricate amphiphilic copolymers based on Poly(ethylene glycol) methyl ether acrylate (PEGA) by Atom Transfer Radical Polymerization (ATRP). AP(PEGA) homopolymer, a block copolymer with styrene PS-bP(PEGA), and an analogous terpolymer including also sodium methacrylate (MANa) in the poly(PEGA) (PPEGA) block, PS-bP(PEGA-co-MANa) have been prepared and characterized to be applied in polymer flooding. Sun et al 37 synthesize a novel amphiphilic polymer based on radical copolymerization of acrylamide, dodecyl polyoxyethylene acrylate (DPEA), and N-(1,1,3,3-tetramethyl butyl) acrylamide (TBA), using potassium persulfate-sodium bisulfite as initiator–activator for enhanced oil recovery (EOR). Tian and Xu 38 prepare a copolymer act as a profile control agent via copolymerization of vinyl triethoxy silane (VTEOS) as a temperature tolerant monomer and Isobutane-Ethylhexyl acrylate(2-EHA)as a salt-resistant monomer with acrylamide monomer (AM) during a free radical copolymerization process. Tong et al 39 fabricate mono-dispersed poly (acrylamide-co-sodium acrylate) hydrogel microparticles with a controlled water absorbance capacity in a droplet microfluidic device which can be used for enhanced oil recovery application. Xu et al 40proposed a new polymer synthesized from acrylamide (AM), 2-acrylamido-2-methylpropane sulfonic acid (AMPS) and 10-hydroxydecylmethacrylate. This polymer thickening the formation brine in harsh reservoir conditions and emulsify the crude oil, in such a way that the accumulative oil recovery of polymer floods using this polymer is expected to be far greater than that of a conventional polymer such as HPAM. Zhang et al 41 reported about the radical polymerization of acrylamide, acryloyl morpholine, and 2-acrylamide-2-methyl propane-sulfonate in water initiated with ammonium persulfate or 2,2-azo bis(2 methylpropionamide) dihydrochloride to produce copolymer for enhanced oil recovery (EOR) applications. The categories of different EOR methods comprise;
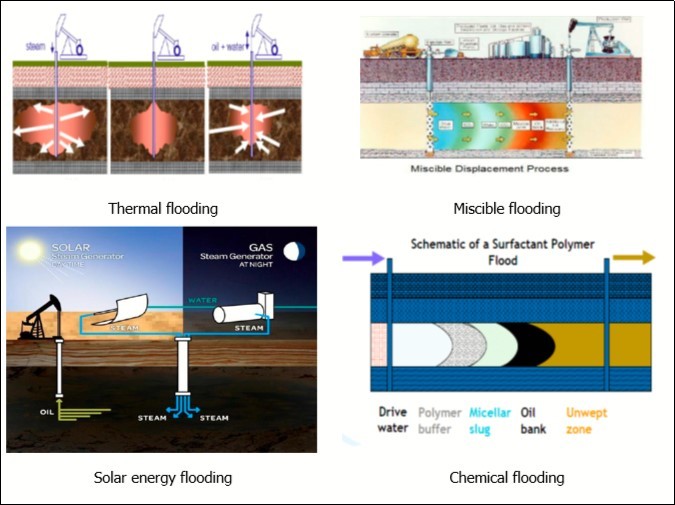
Thermal Flooding
Injection of steam has historically been the most widely applied EOR method. Heat from steam or hot water dramatically reduces heavy oils viscosity, thus improving its flow. The process involves: cyclic steam injection (“huff and puff”, where steam is first injected, followed by oil production from the same well); Continuous steam injection (where steam injected into wells drives oil to separate production wells); hot water injection, and steam assisted gravity drainage (SAGD) using horizontal wells, among others.
Miscible Flooding
Miscible EOR employs supercritical CO2 to displace oil from a depleted oil reservoir. CO2 improve oil recovery by dissolving in, swelling, and reducing the viscosity of the oil. In deep high-pressure reservoirs, compressed nitrogen has been used instead of CO2. Hydrocarbon gases (natural gas and flue gas) have also been used for miscible oil displacement in some large reservoirs. These displacements may simply amount to “pressure maintenance” in the reservoir42. CO2 miscible flooding in crude oil reservoirs is a successful technique to reduce its amount in the atmosphere, in addition to increasing the mobility of the oil and, consequently, increase the reservoir productivity. It is preferred other than hydrocarbon gases since it does not only increase oil recovery but also causes a reduction of greenhouse gas emissions. Moreover, it is a cheap technology as an ultimate long-term geologic storage solution for CO2 owing to its economic productivity from incremental oil production offsetting the cost of carbon sequestration, and exhibit high displacement efficiency and the potential for environmental contamination decrease through its disposal in the petroleum reservoir43.
Chemical Flooding
Chemical flooding involves the injection of an agent not normally present in the reservoir to enhance the oil displacement. The chemical flooding processes involve the injection of three kinds of chemicals; alkaline, surfactant, and polymer (soluble and cross-linked polymers), in addition to other chemicals such as foaming agents, acids and solvents19 and/or combination of alkaline-surfactant-polymer flooding (ASP), and surely the most important substance in these methods is polymer flooding44. In the polymer flooding method, water-soluble polymers aimed to shut-off the high-permeability areas of the reservoir and increase injected water viscosity to increase the swept areas in the reservoir 45 leading to a more efficient displacement of moderately viscous oils. Addition of a surfactant to the polymer formulation may, under very specific circumstances, reduce oil-water interfacial tension and hence remobilizing the trapped oil 46, changing the wettability of the surface, forming emulsions, so enhance the oil production. For some oils, alkaline may convert some naphthenic acids within the oil to surfactants due to the formation of in-situ surfactant. The alkaline may also play a beneficial role in reducing surfactant retention in the rock. For all chemical flooding processes, the inclusion of a viscosifier (usually a water-soluble polymer) is required to provide an efficient sweep of the expensive chemicals through the reservoir.
Other EOR Flooding Processes
Over the years, a number of other innovative EOR processes have been conceived. These methods exhibit varying degrees of promise but require additional development before practical applications. These methods including the following categories;
Injection of Carbonated Water
In which, carbon dioxide (CO2) is dissolved into the water phase before injecting into the reservoir. The dissolved CO2 will transfer from the water phase to the oil phase due to the chemical potential difference of the CO2 in two phases (as a driving force). This interphase mass transfer reduces the oil viscosity, lowers the oil-water interfacial tension (IFT), and causes oil swelling, which will be responsible for the reconnection of isolated residual oil ganglia, mobilizing the trapped oil47.
Microorganisms
application of microorganisms in enhanced oil recovery known as microbially enhanced oil recovery (MEOR). MEOR involves that microbial metabolites are produced in the reservoir rock formation, which makes them more effective. Furthermore, microorganisms metabolize different hydrocarbons at different rates, so boast oil recovery48.
Foams and Other Formulations
Foam is employed to improve the displacement efficiency by which the displacing fluid sweeps the reservoir and increase the recovered oil amount49.
Generally, a flow sheet of EOR processes, worldwide EOR project categories, and different flooding techniques are shown in Figure 1,Figure 2, Figure 3 respectively.
Figure 1.Flow sheet of enhanced oil recovery methods 50.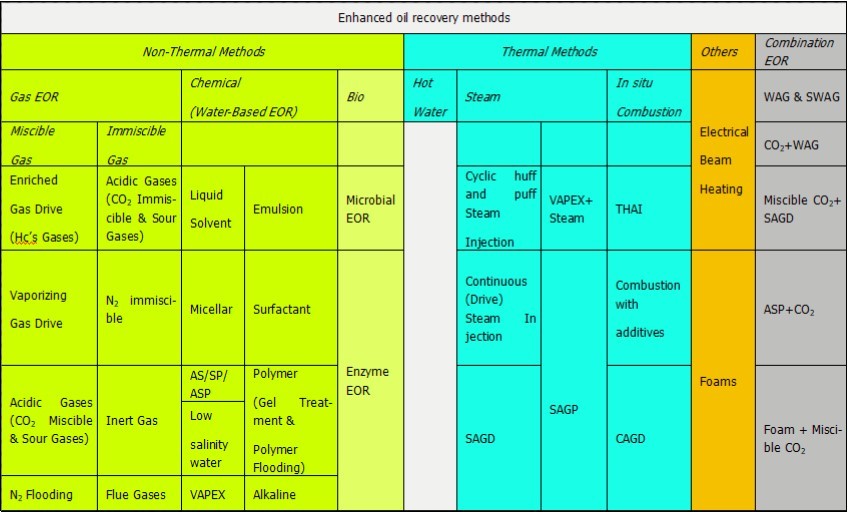
Figure 2.Worldwide EOR project categories, 1959-2010 51.
Properties of Acrylates Polymers
Most polymers used in enhanced oil recovery applications is anionic acrylamide-based polymers which characterized by high molecular weight 4-30 million Dalton and high polydispersity index (PDI) and derived from 3; 1) Copolymerization of acrylamide and sodium acrylate (or/ and sodium Acrylamido-Tertio-Butyl Sulfonate (ATBS)), where copolymerization of acrylamide and sodium acrylate leads to polymer with a more uniform distribution of the anionic charges; 2) Co-hydrolysis or post-hydrolysis of a polyacrylamide; 3) Copolymerization or ter-polymerization of acrylamide with other ionic or non-ionic functional monomers. Polyacrylate polymers exhibit Non- Newtonian behavior (display pseudo plastic criteria) in which viscosity decrease with a shearing increase 30, 31, 32. Since viscosity related to shear rate by the following equation
μ K𝜸 -n …… (Eq1)
Where γ is the shear rate (s–1), µ is the viscosity, cp; K & n are constants. The minus sign indicates the inverse relationship as indicated in Figure 4. Polyacrylamide co-polymers containing a sulfonated monomer are the best candidates for salt tolerance. Under reservoirs conditions, polymers are susceptible to chemical degradation due to the formation of free radicals which attack the polymer chain and result in a reduction of hydrodynamic volume. This chemical degradation occurs owing to; 1)decreasing the intramolecular/intermolecular bindings (hydrogen and van der Waal’s) bonding by electrolytes addition which results in compressing the thicknesses of the electric double layer and hydration layer of the ionic groups, so shield ionic repulsion and causes macromolecular chain contraction with small hydrodynamic volumes, resulting in apparent viscosity reduction; 2) by salinity increase, the solution charge is neutralized, so polymer chain condenses, and viscosity reduction occurs 29, 52. Moreover, increasing of temperature increase polymer hydrolysis degree and generating a higher charge density of anionic functionalities along the polymer backbone. This behavior resort to dissociation of polymer chains specially polyacrylamides in carboxyl groups, which in turn react with divalent cations in the petroleum reservoir. Recently, another range of commercial polymers called associative polymers has been developed to obtain very high resistance factors in the reservoir. These polymers contain both hydrophobic and hydrophilic moieties. The hydrophobic parts can associate in water and provide very high viscosities and resistance factors but with a good propagation in cores 53. More attention has been paid to multifunctional polymers, especially imidazole‐ and imidazoline‐containing polymers which show a wide range of potential applications33 in addition to other vinylated monomers. In recent decades, many studies demonstrated that acrylamide (AM) copolymerized with an applicable functional monomer, such as N, N-dimethylacrylamide, methacrylamide, N-vinyl-2-pyrrolidone (NVP), 2-acrylamido- 2-methyl-1-propane sulfonic acid (AMPS), sodium allyl sulfonate, acrylic acid (AA), and ethylene sulfonic acid, could obtain more satisfying polymer possessing better temperature-resistance and salt-resistance for EOR35. The rheological and viscosity values of polyacrylates are provided in table 1.
Figure 4.Effect of shear rate on viscosity
| Shear rate, S-1 | Apparent Viscosity, cp | Shear Stress, Pa |
| 2.42 | 276.97 | 8.93 |
| 4.62 | 225.20 | 13.87 |
| 6.82 | 198.81 | 18.08 |
| 9.02 | 181.80 | 21.88 |
| 11.22 | 169.54 | 25.38 |
| 13.42 | 160.09 | 28.67 |
| 15.62 | 152.50 | 31.80 |
| 17.82 | 146.21 | 34.78 |
| 20.02 | 140.86 | 37.65 |
| 22.22 | 136.24 | 40.42 |
| 24.42 | 132.18 | 43.11 |
| 26.62 | 128.59 | 45.71 |
| 28.82 | 125.36 | 48.25 |
| 31.02 | 122.44 | 50.73 |
| 33.22 | 119.79 | 53.16 |
| 35.42 | 117.35 | 55.53 |
| 37.62 | 115.11 | 57.86 |
| 39.82 | 113.04 | 60.14 |
| 42.02 | 111.11 | 62.38 |
| 44.22 | 109.31 | 64.59 |
| 46.42 | 107.62 | 66.76 |
| 48.62 | 106.04 | 68.90 |
| 50.82 | 104.55 | 71.01 |
| 53.02 | 103.14 | 73.09 |
| 55.00 | 101.94 | 74.93 |
| 55.00 | 101.94 | 74.93 |
| 55.00 | 101.94 | 74.93 |
| 55.00 | 101.94 | 74.93 |
| 55.00 | 101.94 | 74.93 |
Addition of water-soluble polymer could thicken the formation brine through molecular chain extension, and greatly improve overall oil recovery. This attractive EOR technique referred to as polymer flooding 40. Several publications reported about the synthesis of different hydrophobically associated polyacrylamides (HAPAM) and their modified copolymers, as well as the introduction of special functional groups for improvement of water-solubility, temperature resistance, anti‐shear property, and salt tolerance of partially hydrolyzed polyacrylamide (HPAM). Sulfonate groups attached to the PAM chain can improve effectively the performance on confronting harsh environments like high temperature or high salinity33.
Polymer Flooding Process
Polymer flooding can be carried alone or in combination with other fluids such as alkaline materials for solubilizing the oil and comprise sodium carbonate, caustic soda, borate, and metaborate compounds, silicates, metasilicates, amines, basic polymeric species, or by combination with surfactant molecules3. Polymer flooding can increase recovery up to 5-30% OOIP 54. Polymer flooding process involves the injection of polymer “slug” followed by continued long-term water flooding to drive the polymer slug and the oil bank in front of it toward the production wells as shown in Figure 5. When water is injected into a reservoir, it seeks the path of least resistance (usually the layers of highest permeability) to the lower pressure region of the offset producing wells. If the oil in place has a higher viscosity than the injected water, the water will finger through this oil and result in a low sweep efficiency, or bypassed oil. Flooding tests generated according to the following steps; 1) The sand was firstly cleaned and evacuated then saturated by brine for 14 days followed by oil saturation, then brine flooded until oil cut ceased (i.e. oil cut <1%) and the residual oil amount calculated; 2) the polymer solution with different slug concentrations was flooded at simulated reservoir temperature to determine recovery factor.
Principle and Mechanism of Polymer Displacement
One of the routine screening parameters used for a preliminary analysis of a reservoir is the mobility ratio that represents effects of relative permeability and viscosity of water and oil on mobility based on Darcy’s Law 44.
……. (1)
Where
| M | Mobility ratio |
 |
Water phase mobility |
 |
Oil phase mobility |
 |
Relative permeability of water phase, MD |
 |
Relative permeability of oil phase, MD |
 |
Water phase viscosity, cp |
 |
Oil phase viscosity, cp |
Mobility ratio (M) is defined as mobility of the displacing phase divided by the mobility of the displaced phase. Water-soluble polymer reduce water mobility through different mechanisms: (1) increase the water phase viscosity; 2) reduce the relative permeability of water to the porous rock by adsorption/retention of the polymer in the rock pore throats56 and thereby creating a more efficient and uniform front to displace unswept oil from the reservoir; 3) Another parameter is the viscoelasticity associated with the use of high Molecular weight (Mw) or associative polymers which will recover additional entrapped oil compared to a conventional Newtonian fluid injection3, 57. With a reduced mobility ratio, the sweeping efficiency is increased and, as a consequence, oil recovery is enhanced58. If the mobility ratio (M ≤ 1), the sweeping and displacement efficiencies of the oil by the water phase will be efficient and pistons like fashion 59. By contrast, if the mobility ratio (M > 1), is considered “unfavorable” and unstable because the more mobile displacing fluid will flow more readily than the displaced fluid, and lead to serious viscous fingerings or channel through the oil causing “breakthrough” leaving behind regions of unswept oil. As a result, some of the residual oil is bypassed resulting in poor recovery60. There is also a secondary effect, in which the polymer restores part of the reservoir pressure after its passage (residual resistance factor) 58. This occurs because the polymer builds up a resistance to flow in the portions of the reservoir it penetrates, due to the selective reduction of relative permeability that results from its adsorption58. This increased resistance to flow diverts subsequently injected water into poorly swept areas. Polymer adsorption reduces the relative permeability to the wetting phase more than the relative permeability to the non-wetting phase in water-wet reservoirs 58, 61. The reduction of the relative permeability to water is due to the improved competitive effects of both pore-size restriction and wettability brought about by polymer adsorption58. The most widely used polymers in enhanced oil recovery involve partially hydrolyzed polyacrylamides (HPAM) and hydrophobically associated polyacrylamides (HAPAM).
Partially Hydrolyzed polyacrylamide (HPAM)
HPAM consists of a large proportion of intermediate hydrophilic amide groups and hydrophilic carboxyl groups. Both amide groups and carboxyl groups are linearly introduced onto the backbone of polymeric chains, providing HPAM with favorable solubility and desirable viscosity in fresh water and low salinity formation water 36. HPAM is a synthetic linear chain copolymer of acrylamide and acrylate monomers 62. They are flexible chain appears as a random coil and since it is a polyelectrolyte, it will interact with ions in solution. The main polyacrylamides used are anionic in nature3. Many key aspects need to be considered for the design of a polymer flood such as reservoir characteristics (lithology, stratigraphy, fractures), distribution of remaining oil, well pattern and spacing, polymer degradation, rheology of the polymer solution, compatibility with other chemicals, cost effectiveness 3. HPAM show good viscosifying properties, available in various molecular weights up to 30 million Dalton of relatively low price and can be used for temperatures up to 99 oC depending on brine hardness, so used currently in EOR applications. It is produced generally as free-flowing powders or as self-inverting emulsions. Some experiences reported that acrylamide-based polymers have some disadvantages, such as easy hydrolysis, easy adsorption in the stratum, poor shear stability and poor thermal stability38, and can be summarized as follow;
High sensitivity to thermal, mechanical and chemical degradation at high temperatures) typically above 200°F and exhibit decreasing viscosity by temperature increase63.
Highly susceptible to the presence of oil, surfactant and other reservoir chemicals.
Precipitation can occur if Ca2+or Mg2+is present in the water also, their carboxyl groups are screened by positive ions, leading to reduced viscosity and a limited application in wells with high temperature and salinity64 due to ionic groups shielding, which reduces repulsion and causes chain contraction.
Undergoes molecular weight degradation upon heating in oxygen presence 65.
Respond to physical or chemical stimuli, such as temperature, solvent, mechanical stress, radiation (UV, visible light), and ionic strength 66.
In polymer flooding, the ratio Rh/Rg [pore throat radius (Rh) to gyration radius (Rg)] of a polymer chain should be greater than five67 so, HPAM cause pore throat plugging after a given time68 because of its large molecular size69.
A comparison of different flooding scenarios indicating the cumulative oil recovery, resistance factor and residual resistance factor are summarized in table 2.
Table 2. Comparison of the different flooding reports| Reported works | Cumulative oil recovery | Resistance factor | Residual resistance factor | Applied polymer type |
| Qiao and Zhu70 | 72.8% | × | …. | Modified cationic starch |
| Ghosh et al71 | × | × | 0.96 – 1.22 | Polyacrylamide composite |
| Lai et al72 | × | 11.8 | 2.4 | hyperbranched polyacrylamide polymer |
| Salehi et al73 | × | × | 1.98 | Sulfonated polyacrylamide hydrogels |
| Singh and Mahto74 | × | 14.21 | 1.11 | Polyacrylamide graft starch/clay nanocomposite hydrogel |
| Chuan et al75 | × | 9.38 | 3.39 | surfmer-co-poly acrylates crosslinked hydrogels |
| El-hoshoudy et al76 | 85.35% | 18.3 | 9.0 | Modified cationic starch |
Hydrophobically Associated Polyacrylamide (HAPAM)
Most widely used water-soluble polymers including polyacrylamide and partially hydrolyzed polyacrylamide, are not suitable for high temperature, high salinity, and high flow rate injection owing to hydrolysis, decomposition, degradation, shear damage 35, 62, so these polymers modified by addition of hydrophobic moieties on the backbone structure36 to develop a novel temperature and salinity resistant mobility control agents (polymers) which known as hydrophobically associated polyacrylamides ( HAPAM)77. These polymers class have attracted much attention on both academic and industrial laboratories for polymer flooding in enhanced oil recovery 78, 79 because of their unique structures and properties, including their thickening properties, shear thinning, and anti-polyelectrolyte behavior which has been widely investigated in oil chemistry additives such as mobility control agents and rheology modifiers80. In addition to enhanced thermal stability, relative permeability modifiers, sweeping efficiency, salt-tolerance behavior and high viscosifying properties for IOR or EOR applications81. These polymers synthesized by modification of partially hydrolyzed polyacrylamide (HPAM) through grafting or incorporating hydrophobic chain cross-linking segments onto their hydrophilic main chain 33 or by copolymerization of hydrophilic and hydrophobic monomers 82. They considered as promised EOR candidates for polymer flooding in high salinity reservoirs, owing to their unique characteristics83 which can be summarized as follow;
In aqueous solutions, above a critical association concentration (C∗), their hydrophobic groups develop intermolecular hydrophobic associations in nanodomains, leading to building up of a 3D-transient network structure in high ionic strength medium 84 so, providing excellent viscosity building capacity 79, 85, 86, remarkable rheological properties and better stability with respect to salts than the unmodified HPAM precursors 87.
Reduce interfacial tension at the solid/liquid interface, since hydrophobic moieties associates forming aggregates or micelles.
Shows an unusual adsorption isotherm88 so, can be considered as a wettability modifier.
Does not undergo mechanical degradation under high shear stress such as those encountered in pumps and near the wellbore area, since the physical links between chains are disrupted before any irreversible degradation occurs, also they reform and retain their viscosity upon shear decreasing 89.
Highly resistance to physicochemical conditions (temperature, pH, and ion content) prevailing around the wells, so considered a prospective EOR candidate as thickeners or rheology modifiers in high temperature, high-pressure reservoirs 90, 91, 92, reservoir stimulation93 and tertiary oil recovery 94.
Hydrophobically associating polyacrylamide are prepared conveniently by a micellar polymerization method, a lot of small molecule surfactants need to be added in order to enable hydrophobic monomer to be solubilized into micelles, and the addition of small molecule surfactants brings many negative influences 95. When hydrophilic surfmers are adopted to prepare HAPAM, homogeneous phase copolymerization of hydrophilic surfmers and acrylamide in aqueous solution can be carried out because of their solubility in water, and those drawbacks caused by the addition of small molecule surfactants can be avoided completely. Furthermore, when the concentration of surfmers is above their critical micellar concentration (CMC), their copolymerization with acrylamide follows the macroblock copolymerization mechanism, namely, surfmer molecules will incorporate into the macromolecular main chains as macroblock form. The macroblock macromolecular structure leads to more effectively hydrophobic associating of the hydrophobic side chains, stronger thickening property of HAPAM 81 and improved salinity resistance of HAPAM. Surfmers can copolymerize with the main monomer and they become covalently bound to particles surface forming integral polymeric materials. As a result, desorption of surfactant from the polymer particles or migration in the polymer films is impeded. Such improvements of latex and/or polymer stability properties96 have been reported for mechanical stability, electrolyte stability of the latex97, a decrease of surfactant migration98 and control of surface charge density99.
Conclusion
Enhanced oil recovery is a widely implemented technology nowadays due to a sustained energy crisis associated with overgrowing human civilization. These aspects impose on chemists and scientists to discover and develop novel materials and techniques to maximize trapped oil in the reservoirs. One of these developed technologies is polymer flooding through copolymerization of different acrylate monomers which can withstand salinity, temperature, PH and bacterial degradation, in addition to increasing water phase viscosity. In this work, properties of acrylate polymers, as well as rheological and solution properties, were investigated at simulated reservoir conditions of high salinity and temperature. Moreover, the mechanism and techniques of polymer flooding were considered. On the other hand, a comparison between hydrolyzed and hydrophobically associated polymers was evaluated with respect to EOR applications. Several authors do their best effort in this field and already synthesize different polymers, but the increased energy demand impose more and more research on these applications.
References
- 1.Raffa P, Broekhuis A A, Picchioni F. (2016) Amphiphilic copolymers based on PEG‐acrylate as surface active water viscosifiers: Towards new potential systems for enhanced oil recovery. , Journal of Applied Polymer Science 133(42).
- 2.Pu W-F, Liu R, Wang K-Y, Li K-X, Yan Z-P et al. (2015) Water-soluble core–shell hyperbranched polymers for enhanced oil recovery. , Industrial & Engineering Chemistry Research 54(3), 798-807.
- 3.Thomas A, Gaillard N, Favero C. (2012) Some key features to consider when studying acrylamide-based polymers for chemical enhanced oil recovery. Oil & Gas Science and Technology–Revue d’IFP Energies Nouvelles 67(6), 887-902.
- 4.ANMB El-hoshoudy. (2018) Emulsion Polymerization Mechanism. Recent Research in Polymerization. InTech;.
- 5.El-Hoshoudy A, Desouky S, Elkady M, Alsabagh A, Betiha M et al.Investigation of optimum polymerization conditions for synthesis of cross-linked polyacrylamide-amphoteric surfmer nanocomposites for polymer flooding in sandstone reservoirs. , International Journal of Polymer Science 2015-2015.
- 6.El-hoshoudy A, Desouky S, Elkady M, Alsabagh A, Betiha M et al. (2016) Hydrophobically associated polymers for wettability alteration and enhanced oil recovery–Article review. , Egyptian Journal of Petroleum
- 7.Green D W, Willhite G P. (1998) Enhanced oil recovery.Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers. , Richardson, TX;
- 8.Chukwudeme E A, Fjelde I, Abeysinghe K P, Lohne A. (2011) Effect of interfacial tension on water/oil relative permeability and remaining saturation with consideration of capillary pressure.SPE. EUROPEC/EAGE Annual Conference and Exhibition.Society of Petroleum Engineers; .
- 9.Dabbous M K. (1977) Displacement of polymers in waterflooded porous media and its effects on a subsequent micellar flood. , Society of Petroleum Engineers Journal 17(05), 358-68.
- 10.Platt Jr JL. (1981) Poly-(alpha-alkoxy) acrylamide and poly-(alpha-alkoxy) acrylamide complexes. Google Patents;.
- 11.Hunter T N, Pugh R J, Franks G V, Jameson G J. (2008) The role of particles in stabilising foams and emulsions. Advances in colloid and interface science 137(2), 57-81.
- 12.Cao R, Cheng L, Lian P. (2015) Flow behavior of viscoelastic polymer solution in porous media. , Journal of Dispersion Science and Technology 36(1), 41-50.
- 14.McCormick C L, Chen G S, Hutchinson B H. (1982) Water‐soluble copolymers. V. Compositional determination of random copolymers of acrylamide with sulfonated comonomers by infrared spectroscopy and C13 nuclear magnetic resonance. , Journal of Applied Polymer Science 27(8), 3103-20.
- 15.Wan W-M, Pickett P D, Savin D A, McCormick C L. (2014) Structurally controlled “polysoaps” via RAFT copolymerization of AMPS and n-dodecyl acrylamide for environmental remediation. , Polymer Chemistry 5(3), 819-27.
- 16.Martin F, Hatch M, Shepitka J, Ward J. (1983) Improved water-soluble polymers for enhanced recovery of oil.SPE oilfield and geothermal chemistry symposium.Society of Petroleum Engineers;.
- 17.Osterloh W, Jante Jr M. (1992) Surfactant-polymer flooding with anionic PO/EO surfactant microemulsions containing polyethylene glycol additives.SPE/DOE Enhanced Oil Recovery Symposium.Society of Petroleum Engineers;.
- 18.Thomas S, Ali S, Scoular J, Verkoczy B. (2001) Chemical methods for heavy oil recovery. , Journal of Canadian Petroleum Technology 40(03).
- 19.Schramm L L. (2000) Surfactants: fundamentals and applications in the petroleum industry.Cambridge.
- 20.Du Y, Guan L. (2004) Field-scale polymer flooding: lessons learnt and experiences gained during past. 40 years.SPE International Petroleum Conference in Mexico.Society of Petroleum Engineers; .
- 21.MMM A N El-hoshoudy, S M Desouky, Ramzi M. (2019) Experimental, modeling and simulation investigations of a novel surfmer-co-poly acrylates crosslinked hydrogels for water shut-off and improved oil recovery. , Journal of Molecular Liquids 277, 142-56.
- 22.SMD a n el-hoshoudy. (2018) Synthesis and evaluation of acryloylated starch-g-poly (Acrylamide/ Vinylmethacrylate/1-Vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent. , international Journal of Biological Macromolecules 116, 434-42.
- 23.SMD a n el-hoshoudy, A M. (2018) Synthesis of starch functionalized sulfonic acid co-imidazolium/silica composite for improving oil recovery through chemical flooding technologies. , International Journal of Biological Macromolecules
- 24.El-hoshoudy A, Desouky S, Alsabagh A, Betiha M, E-k M Y et al. (2017) Evaluation of solution and rheological properties for hydrophobically associated polyacrylamide copolymer as a promised enhanced oil recovery candidate. , Egyptian Journal of Petroleum
- 25.El-hoshoudy A, Desouky S, Elkady M, Alsabagh A, Betiha M et al. (2017) Hydrophobically associated polymers for wettability alteration and enhanced oil recovery–Article review. , Egyptian Journal of Petroleum
- 26.an el-hoshoudy. (2018) Quaternary ammonium based surfmer-co-acrylamide polymers for altering carbonate rock wettability during water flooding. , Journal of Molecular Liquids
- 27.el-hoshoudy A N. (2019) Synthesis of acryloylated starch-g-poly acrylates crosslinked polymer functionalized by emulsified vinyltrimethylsilane derivative as a novel EOR agent for severe polymer flooding strategy. , International journal of Biological Macromolecules 123, 124-32.
- 28.El-hoshoudy AN DS, Attia A M, G S. (2018) Synthesis and Evaluation of Xanthan-G-Poly (Acrylamide) CoPolymer for Enhanced Oil Recovery Applications. , Petroleum & Petrochemical Engineering Journal 2(3).
- 29.El-Hoshoudy A, Desouky S, Betiha M, Alsabagh A. (2016) Use of 1-vinyl imidazole based surfmers for preparation of polyacrylamide–SiO2 nanocomposite through aza-Michael addition copolymerization reaction for rock wettability alteration. , Fuel 170, 161-75.
- 30.El-hoshoudy A. (2018) Quaternary ammonium based surfmer-co-acrylamide polymers for altering carbonate rock wettability during water flooding. , Journal of Molecular Liquids 250, 35-43.
- 31.El-Hoshoudy A, Desouky S. (2018) Synthesis and evaluation of acryloylated starch-g-poly (Acrylamide/Vinylmethacrylate/1-Vinyl-2-pyrrolidone) crosslinked terpolymer functionalized by dimethylphenylvinylsilane derivative as a novel polymer-flooding agent. , International journal of biological macromolecules 116, 434-42.
- 32.El-Hoshoudy A, Desouky S, Attia A. (2018) Synthesis of starch functionalized sulfonic acid co-imidazolium/silica composite for improving oil recovery through chemical flooding technologies. International journal of biological macromolecules.
- 33.Gou S, Luo S, Liu T, Xia H, Jing D et al. (2015) Thermally stable imidazoline-based sulfonate copolymers for enhanced oil recovery. , RSC advances 5(104), 85165-73.
- 34.Hsieh H, Moradi‐Araghi A, Stahl G, Westerman I. (1992) Water‐soluble polymers for hostile environment enhanced oil recovery applications.MakromolekulareChemie. Macromolecular Symposia.64, Wiley Online Library 121-35.
- 35.Lai N, Qin X, Ye Z, Peng Q, Zhang Y et al.Synthesis and evaluation of a water-soluble hyperbranched polymer as enhanced oil recovery chemical. , Journal of Chemistry 2013-2013.
- 36.Liu R, Pu W, Sheng J J, Du D. (2017) Star-like hydrophobically associative polyacrylamide for enhanced oil recovery: comprehensive properties in harsh reservoir conditions. , Journal of the Taiwan Institute of Chemical Engineers 80, 639-49.
- 37.Sun J, Xu X, Wang J, Zhang W, Yang H et al. (2010) Synthesis and emulsification properties of an amphiphilic polymer for enhanced oil recovery. , Journal of Dispersion Science and Technology 31(7), 931-5.
- 38.Tian M, Xu Y J. (2013) The Preparation and Characterization of AM/2-EHA/VTEOS Copolymer as Profile Control Agent.Advanced Materials Research.602. Trans Tech Publ;. 732-8.
- 39.Tong D, Yesiloz G, Ren C L. (2017) Madhuranthakam CMR. Controlled Synthesis of Poly (acrylamide-co-sodium acrylate) Copolymer Hydrogel Microparticles in a Droplet Microfluidic Device for Enhanced Properties. , Industrial & Engineering Chemistry Research 56(51), 14972-9.
- 40.Xu X, Ouyang J, Wang Y, Wang C. (2017) Experimental investigation using an acrylamide-based polymer with emulsifying capability for enhanced oil recovery: A preliminary study. , Journal of Industrial and Engineering Chemistry 55, 110-8.
- 41.Zhang P, Bai S, Chen S, Li D, Jia Z et al. (2018) Preparation, solution characteristics and displacement performances of a novel acrylamide copolymer for enhanced oil recovery (EOR). , Polymer Bulletin 75(3), 1001-11.
- 42.A N El-hoshoudy, Desouky S M. (2016) Hydrophobic Polymers for Enhanced Oil Recovery: Preparation, Characterization, Rheological Properties.
- 43.El-Hoshoudy A N, Desouky S. (2018) CO2 Miscible Flooding for Enhanced Oil Recovery. Carbon Capture, Utilization and Sequestration. IntechOpen;.
- 44.Abidin A, Puspasari T, Nugroho W. (2012) Polymers for enhanced oil recovery technology. , Procedia Chemistry 4, 11-6.
- 46.Chen H, Lucas L, Nogaret L, Yang H, Kenyon D. (2000) Laboratory monitoring of surfactant imbibition using computerized. tomography.SPE International Petroleum Conference and Exhibition in Mexico.Society of Petroleum Engineers; .
- 47.Esene C, Rezaei N, Aborig A, Zendehboudi S. (2019) Comprehensive review of carbonated water injection for enhanced oil recovery. , Fuel 237, 1086-107.
- 48.Lazar I, Petrisor I, Yen T. (2007) Microbial enhanced oil recovery (MEOR). , Petroleum Science and Technology 25(11), 1353-66.
- 49.Sunmonu R M, Onyekonwu M. (2013) Enhanced oil recovery using foam injection; a mechanistic approach.SPE. Nigeria Annual International Conference and Exhibition.Society of Petroleum Engineers; .
- 50.Mai A, Kantzas A. (2009) Heavy oil waterflooding: effects of flow rate and oil viscosity. , Journal of Canadian Petroleum Technology 48(03), 42-51.
- 52.Dai Y-h, Wu F-p, Li M-z, Wang E-j. (2008) Properties and influence of hydrophobically associating polyacrylamide modified with 2-phenoxylethylacrylate. , Frontiers of Materials Science in China 2(1), 113-8.
- 53.Seright R S, Fan T, Wavrik K E, Wan H, Gaillard N et al. (2011) Rheology of a new sulfonic associative polymer. in porous media.SPE International Symposium on Oilfield Chemistry.Society of Petroleum Engineers; .
- 54.Pope G A. University of Texas at (2007) Overview of chemical EOR.Casper EOR Workshop.The. , Austin Austin, TX;
- 55.El-hoshoudy A N, Desouky S M, Betiha M H, Alsabagh A M. (2017) Hydrophobic Polymers Flooding. Application and Characterization of Surfactants. IntechOpen;.
- 57.Ezell R G, McCormick C L. (2007) Electrolyte‐and pH‐responsive polyampholytes with potential as viscosity‐control agents in enhanced petroleum recovery. , Journal of applied polymer science 104(5), 2812-21.
- 58.Zheng C, Gall B, Gao H, Miller A, Bryant R. (1998) Effects of polymer adsorption and flow behavior on two-phase flow in porous.SPE/DOE Improved Oil Recovery Symposium.Society of Petroleum Engineers;.
- 59.Mukherjee S, Das S S, Dhar J, Chakraborty S, DasGupta S. (2017) Electroosmosis of viscoelastic fluids: Role of wall depletion layer. , Langmuir 33(43), 12046-55.
- 60.Seright R S. (2005) Clean up of oil zones after a gel treatment.SPE International Symposium on Oilfield Chemistry.Society of Petroleum Engineers;.
- 61.Ali L, Barrufet M. (2001) Using centrifuge data to investigate the effects of polymer treatment on relative permeability. , Journal of Petroleum Science and Engineering 29(1), 1-16.
- 62.Mansour A M, Al-Maamari R S, Al-Hashmi A S, Zaitoun A, Al-Sharji H. (2014) In-situ rheology and mechanical degradation of EOR polyacrylamide solutions under moderate shear rates. , Journal of Petroleum Science and Engineering 115, 57-65.
- 63.Manafi M, Manafi P, Agarwal S, Bharti A K, Asif M et al. (2017) Synthesis of nanocomposites from polyacrylamide and graphene oxide: application as flocculants for water purification. Journal of colloid and interface science 490:. 505-10.
- 64.Silva C C, Farias J P, de Sant’Ana HB. (2009) Evaluation of AISI 316L stainless steel welded plates in heavy petroleum environment. , Materials & Design 30(5), 1581-7.
- 65.Wei B. (2015) Flow characteristics of three enhanced oil recovery polymers in porous media. , Journal of Applied Polymer Science 132(10).
- 66.Tanaka T. (2000) Experimental methods in polymer science: modern methods in polymer research and technology.Academic Press;.
- 67.Yiqiang L, Junxin G, Dandan Y, Junjian L, Hualong L.Study on the matching relationship between polymer hydrodynamic characteristic size and pore throat radius of target block S based on the microporous membrane filtration method. , Journal of Chemistry 2014-2014.
- 68.Shi J, Varavei A, Huh C, Delshad M, Sepehrnoori K et al. (2011) Transport model implementation and simulation of microgel processes for conformance and mobility control purposes. , Energy & Fuels 25(11), 5063-75.
- 69.Zhong C, Luo P, Ye Z, Chen H. (2009) Characterization and solution properties of a novel water-soluble terpolymer for enhanced oil recovery. , Polymer Bulletin 62(1), 79-89.
- 70.Qiao R, Zhu W. (2010) Evaluation of modified cationic starch for impeding polymer channeling and in-depth profile control after polymer flooding. , Journal of Industrial and Engineering Chemistry 16(2), 278-82.
- 71.Ghosh B, Bemani A, Wahaibi Y, Hadrami H, Boukadi F H. (2012) Development of a novel chemical water shut-off method for fractured reservoirs: Laboratory development and verification through core flow experiments. , Journal of Petroleum Science and Engineering 96, 176-84.
- 72.Lai N, Qin X, Ye Z, Li C, Chen K et al. (2013) The study on permeability reduction performance of a hyperbranched polymer in high permeability porous medium. , Journal of Petroleum Science and Engineering 112, 198-205.
- 73.Salehi M B, Vasheghani‐Farahani E, Sefti M V, Moghadam A M, Naderi H. (2014) Rheological and transport properties of sulfonated polyacrylamide hydrogels for water shutoff in porous media. Polymers for Advanced Technologies. 25(4), 396-405.
- 74.Singh R, Mahto V. (2017) Synthesis, characterization and evaluation of polyacrylamide graft starch/clay nanocomposite hydrogel system for enhanced oil recovery. , Petroleum Science 14(4), 765-79.
- 75.Yang-Chuan K, Guang-Yao W, Yi W. (2008) Preparation, morphology and properties of nanocomposites of polyacrylamide copolymers with monodisperse silica. , European Polymer Journal 44(8), 2448-57.
- 76.El-hoshoudy A, Mohammedy M, Ramzi M, Desouky S, Attia A. (2019) Experimental, modeling and simulation investigations of a novel surfmer-co-poly acrylates crosslinked hydrogels for water shut-off and improved oil recovery. , Journal of Molecular Liquids 277, 142-56.
- 77.Zaitoun A, Makakou P, Blin N, Al-Maamari R S, A-AR Al-Hashmi et al. (2012) Shear stability of EOR polymers. , Spe Journal 17(02), 335-9.
- 78.Bastiat G, Grassl B, François J. (2002) Study of sodium dodecyl sulfate/poly (propylene oxide) methacrylate mixed micelles for the synthesis of thermo‐associative polymers by micellar polymerization. , Polymer international 51(10), 958-65.
- 79.Feng Y, Billon L, Grassl B, Bastiat G, Borisov O et al. (2005) Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. 2. Properties of non-hydrolyzed polymers in pure water and brine. , Polymer 46(22), 9283-95.
- 80.Zhao Y, Zhou J, Xu X, Liu W, Zhang J et al. (2009) Synthesis and characterization of a series of modified polyacrylamide. , Colloid and Polymer Science 287(2), 237-41.
- 81.Gao B, Wu N, Li Y. (2004) Study on tercopolymer of acrylamide containing strong anions and hydrophobic association blocks. , Acta Polymerica Sinica 5, 736-42.
- 82.Camail M, Margaillan A, Martin I. (2009) Copolymers of N‐alkyl‐and N‐arylalkylacrylamides with acrylamide: influence of hydrophobic structure on associative properties. Part I: viscometric behaviour in dilute solution and drag reduction performance. , Polymer International 58(2), 149-54.
- 83.Niu Y, Jian O, Zhu Z, Wang G, Sun G. (2001) Research on hydrophobically associating water-soluble polymer used for EOR.SPE International Symposium on Oilfield Chemistry.Society of Petroleum Engineers;.
- 84.Candau F, Selb J. (1999) Hydrophobically-modified polyacrylamides prepared by micellar polymerization1. Advances in colloid and interface science. 79(23), 149-72.
- 85.Shashkina Y A, Zaroslov Y D, Smirnov V, Philippova O, Khokhlov A et al. (2003) Hydrophobic aggregation in aqueous solutions of hydrophobically modified polyacrylamide in the vicinity of overlap concentration. , Polymer 44(8), 2289-93.
- 86.Gao B, Wu N, Li Y. (2005) Interaction between the strong anionic character of strong anions and the hydrophobic association property of hydrophobic blocks in macromolecular chains of a water‐soluble copolymer. , Journal of applied polymer science 96(3), 714-22.
- 87.Maia A M, Borsali R, Balaban R C. (2009) Comparison between a polyacrylamide and a hydrophobically modified polyacrylamide flood in a sandstone core. , Materials Science and Engineering: C 29(2), 505-9.
- 88.Hu S-S, Zhang L, Cao X-L, Guo L-L, Zhu Y-W et al. (2015) Influence of crude oil components on interfacial dilational properties of hydrophobically modified polyacrylamide. , Energy & Fuels 29(3), 1564-73.
- 89.Evani S, Rose G. (1987) Water soluble hydrophobe association polymers. , Polym Mater Sci Eng 57, 477-81.
- 90.JdA Rodrigues, Lachter E R, de Sá CH, M de, RSV Nascimento. (2006) New multifunctional polymeric additives for water-based muds.SPE. Annual Technical Conference and Exhibition.Society of Petroleum Engineers; .
- 91.Gouveia L M, Paillet S, Khoukh A, Grassl B, Müller A J. (2008) The effect of the ionic strength on the rheological behavior of hydrophobically modified polyacrylamide aqueous solutions mixed with sodium dodecyl sulfate (SDS) or cetyltrimethylammonium p-toluenesulfonate (CTAT). Colloids and Surfaces A: Physicochemical and Engineering Aspects. 322(13), 211-8.
- 92.Zhong C, Huang R, Xu J. (2008) Characterization, solution behavior, and microstructure of a hydrophobically associating nonionic copolymer. , Journal of Solution Chemistry 37(9), 1227-43.
- 93.Dalrymple E D, Eoff L S, Everett D M. (2008) Conformance while fracturing tight gas formations.SPE Tight Gas Completions Conference.Society of Petroleum Engineers;.
- 94.Ranjbar M, Schaffie M. (2000) Improved treatment of acrylamide co-and terpolymers for water control in gas-producing and storage wells. , Journal of Petroleum Science and Engineering 26(1), 133-41.
- 95.Guyot A. (2004) Advances in reactive surfactants. Advances in Colloid and Interface Science 108, 3-22.
- 97.Atta A M, Dyab A K, Al-Lohedan H A, AlJenady K A. (2014) Novel reactive polymerizable nonyl phenol ethoxylate surfactants as emulsifier in non-aqueous emulsion polymerization. , Polymer Science Series B 56(6), 770-87.
Cited by (12)
This article has been cited by 12 scholarly works according to:
Citing Articles:
Petroleum Science and Technology (2025) OpenAlex
ACS Omega (2025) OpenAlex
Avantika Kaushik, D. Joshi, Rohit Kumar Saw, Kiran Bala Rathi, Sujit Mitra et al. - Fuel (2024) Semantic Scholar
(2023) OpenAlex
Fuel (2023) OpenAlex
A. N. El-hoshoudy - The Arabian journal for science and engineering (2022) Semantic Scholar
Gameil Sameh, Mohamed Ismaiel, A. Fakhry, Omar Hesham, Felopateer Magdy et al. - Journal of Chemistry and Applications (2021) Semantic Scholar
Petroleum & Petrochemical Engineering Journal (2021) OpenAlex
Kelly Lúcia Nazareth Pinho de Aguiar, L. Palermo, C. Mansur - Oil & Gas Science and Technology (2021) Semantic Scholar
Oil & Gas Science and Technology – Revue d’IFP Energies nouvelles (2021) OpenAlex
Petroleum & Petrochemical Engineering Journal (2019) OpenAlex
Elizariev An - Petroleum & Petrochemical Engineering Journal (2019) Semantic Scholar