Abstract
Percutaneous device closure of atrial septal defects (ASD) has proven to be safe and it is nowadays the standard treatment for ASDs. Immediate or late device embolization is a rare but potential complication of every attempted ASD device closure. We report a case of asymptomatic Amplatzer Septal Occluder into the left ventricular outflow tract (LVOT) detected by routine transthoracic echocardiography 3 months after successful implantation in a stable patient.
Author Contributions
Academic Editor: Demirozu ZT, Deparment of Cardiovascular Surgery, Koc University Hospital, Istanbul, Turkey.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Sergiu Vijiala, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Atrial septal defect (ASD) is the fourth most common congenital heart defect, with an incidence of 2.78 per 10,000 live births. 1 The gold standard treatment of ASD since 1960s was surgery with good postoperative results on the long-term follow-up. In 1975 King&Mills performed the first application of ASD closure in the human population using a transvenous umbrella during cardiac catheterization.2 Percutaneous atrial septal defect closure using the Amplatzer septal occluder (ABBOTT) or many other devices is an established alternative treatment to the classical surgery with excellent safety, clinical outcome, being an effective procedure. In the last 2 decades, this technique replaced surgical closure of ASDs in most centres, becoming a widely accepted and practiced procedure. With the accelerated growth in transcatheter device closure, many centres started to report outcome data for this procedure with a general interest focused on its complications. Late complications are more common with the advances in the procedure and the rate of immediate complication is very low. The need for immediate surgery following the implantation is rare (<1%) as reported by Godart et al. in 2015. 5
Procedure Related Complications
As in any interventional cardiac catheterisation procedure, percutaneous transcatheter ASD closure is associated with all the general risks, the most common and frequent immediate complications related to the transcatheter itself being vessel or cardiac perforation, the introduction of an infectious pathogen and the risk of contrast agent reactions. Spence and col. reported in 2005 in Heart that the complications of femoral vein access like haematomas rarely need blood transfusions and less frequently surgical repair when retroperitoneal haematomas developed. 6
The most common reported complication in literature are device embolisation or malposition (3.5%), followed (not in the frequency order, but rather randomly because the rate and type of complications depend on the experience of the centres and patients particularities) by arrhythmia such as atrial fibrillation requiring electrical cardioversion most of the time, transient atrioventricular block, transient ST elevation, deep vein thrombosis, pericardial effusion (most of the time from cardiac perforation), thrombus formation on the atrial disc of the occluder device, air embolism to the right heart after device implantation and transient ischaemic attack. A couple of isolated reports of systemic allergic reaction to nickel-containing atrial septal closure devices are found in the literature. 7, 8, 9 In total, there are at least 29 reported complications with Amplatzer septal occluder device that were reviewed by Divekar et al. 15.
A rare complication of the percutaneous closure of ASD is cardiac perforation. During device implantation, the guidewire, sheath, or device may cause the perforation of the left atrial wall, pulmonary vein, or right atrium. Most cardiac perforations occur 3 days after the procedure and are associated with hemopericardium or tamponade. 4
Amin et al. reported some very serious complications of cardiac erosion by the Amplatzer septal occluder after catheter closure of ASDs. Erosions have been identified by the late development of pericardial effusion or even tamponade. Data were collected from 28 cases worldwide and all the erosions occurred near the aortic root. 10
During the initial hours to the first 24 h device embolization is more frequently encountered, but can also occur late after implantation, even at 12 months as in a case reported by Kim. 3 Patients develop symptoms depending upon the chamber to which the device is migrated. In the patients in whom the devices embolised or are malpositioned, the surgical retrieval and the catheter techniques retrieval are the two options of treatment.
Case Report
A 58 year old woman was referred to our cardiology department for elective ASD device closure due to a moderate left-right shunt with repercussion on the right heart cavities. Communication was detected by transthoracic and transoesophageal echocardiography. It was a type ostium primum ASD located in the posterior and upper part of the interatrial septum, without anomaly of the venous return. The dimension of the defect was 12/15 mm with correct anterior, superior and inferior rims, so we decided to close it percutaneously although surgical closure is habitually proposed in ostium primum ASD type. The defect was closed by an Amplatzer ASD occluder of 18 mm without any immediate complication. Transthoracic echocardiography immediately after the procedure (before the discharge) reported a normal position of the device without residual shunt. The next day the patient appeared asymptomatic and in haemodynamically stable condition and could leave the hospital. Our follow-up protocol following the ASD closure stipulates transthoracic echocardiography immediately after the procedure, at 3 months, at 6 months and then yearly or in emergency in cases of symptomatic patients.
Ambulatory routine follow-up transthoracic echocardiography at 3 months revealed embolization of the device in the left ventricular outflow tract (LVOT) and the patient was sent to our department. No cardiovascular or neurological symptoms were related so the time of the embolization could not be precisely ascertained. She also did not have any regular follow up until the 3 months transthoracic echocardiography.
On examination, her heart rate was 71 beats per minute regular, blood pressure was 143/80 mm Hg, air saturation was 96% breathing ambient air and no fever. Jugular veins were not engorged. S1 and S2 were normal. There was a grade 3/6 ejection systolic murmur at right upper parasternal area. 24 hours ECG monitoring showed sinus rhythm. The neurological and pulmonary examination was normal. She underwent immediate transthoracic echocardiography and subcostal view showed a left-right shunt at the interatrial septum. Further evaluation revealed that the device embolized to the left ventricle and was lying in the left ventricular outflow tract with the distal extremity crossing the aortic ring. The device was displaced longitudinally with an important effect of stenosis. (Figure 1), (Figure 2). The entrapped device induced mechanical obstruction of the left ventricular outflow tract with a maximum/minimum gradients of 64/42 mmHg. The left ventricular function and size were normal. The mitral valve was normal with a minimal regurgitation. The right ventricle was mildly enlarged with normal function. The tricuspid valve function was normal. No pericardial effusion was detected. There was no gross thrombus deposition over the device in echography and no vegetation was detected anywhere.
Figure 1.Entraped Amplatzer ASD closure device in the left ventricular outflow tract. LA : left atrium, AO : aorta, arrow : ASD closure device.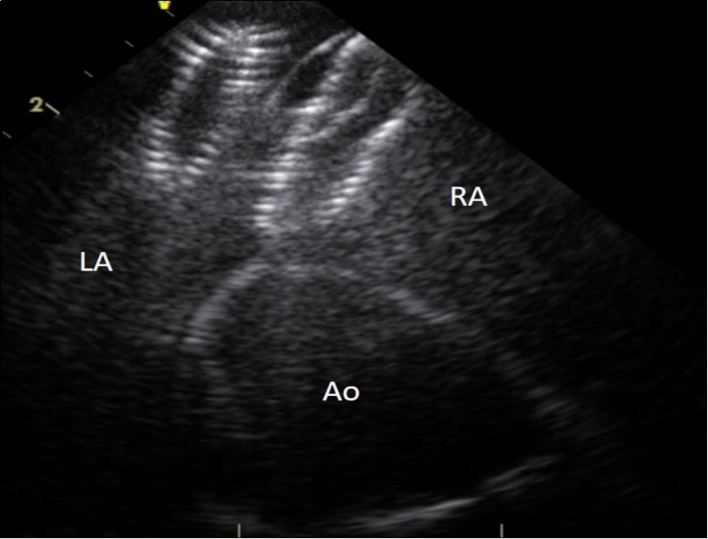
Figure 2.Intracardiac ultrasound picture of the ASD Amplatzer device in place on the interatrial septum. RA :right atrium. LA:left atrium. Ao :aorta
We tried a trans-femoral percutaneous retrieval approach in order to catch the device, but the procedure was complicated by a right ventricle perforation with a haemorrhagic shock. She underwent a pericardiocentesis with Amplatzer retrieval and surgical closure of the ASD. The procedure was further complicated with an atrial fibrillation that was pharmacologically cardioverted. The evolution was without further complications and the patient was dispatched home after 10 days
Discussion
In centres with a lot of expertise, the incidence of atrial septal occluder embolization is estimated as 0.55% to 1.1% with the common causes of embolization being the large defects, larger devices, inadequate rims, undersized device, or insufficient left atrial size to accommodate a device. Deficient rim means any rim width of less than 5 mm in the vicinity of superior vena cava, inferior vena cava, right upper and lower pulmonary veins, coronary sinus, and atrioventricular valves even though this definition is not followed in majority of catheterization laboratories and larger devices are deployed to splay the discs of the device in both sides of the aorta. 11 Larger ASD (> 20 mm) and device size (> 24 mm) seem to be the most predictors factors related to device embolization as several studies reported16.
The dislocated device can migrate to the main pulmonary artery, left ventricle, left atrium, ascending aorta, aortic arch or descending thoracic aorta. Devices usually embolize into the main pulmonary artery (89%). Most migrations (67%) are detected within the first 24 hours and migration to the descending thoracic aorta in the late postoperative period (>1 year) is an extremely rare occurrence as we already mentioned. 3
Very rarely, silent embolization has been reported at one week 12 or even at one 3 or two years after implantation13, but the most common time of embolization is in the first hours after implantation, early complications being accepted by most authors till one or two days.
Probably the easiest complication to detect is left sided embolization since is almost always symptomatic as the dislodged device may obstruct the left ventricular inflow or outflow tract. This leads to symptoms of left heart failure of dyspnoea in different grade. However, as in our case, Errahmouni A et al. reported a case of silent embolization into the left ventricular outflow tract, which was detected in routine echocardiography at one week after the device deployment 14 On the other hand, dislodgement to right side can present with a different symptomatology, depending upon the site of its dislodged position. It seem that acute dislodgement to right ventricle most commonly leads to incessant ventricular arrhythmias and the right ventricular inflow or outflow obstruction may lead to features of right heart failure. Rarely, it seems, embolization to mid cavity of right ventricle may be asymptomatic. 11
In our case, without the follow-up transthoracic echocardiography the patient could have probably remained asymptomatic for a couple of months taking into account that she performed snowshoeing after the Amplatzer Septal Occluder implementation and she was asymptomatic during the effort. This lack of symptoms could be explained partially due to the small size of the Amplatzer. The cause of the migration remains unknown since we cannot established with certitude the moment of the dislocation, but the unusual localisation of the ASD without any posterior and upper rim was certainly the major risk factor of embolisation.
In order to avoid embolization it is probably reasonable to consider a larger device even if the technique could become a little bit more challenging and devices which fit better should be developed.
Conclusion
Further studies on the early and late complications associated with transcatheter occlusion of ASD should evaluate the proper time of the follow up with transthoracic echocardiography since regular follow-up is the only method to detect complications in asymptomatic patients. The follow-up will give us the opportunity to prevent any late embolization or other complications like erosion or perforation leading to tamponade that always require an emergency surgical approach.
References
- 1.Chessa M, Carminati M, Butera G. (2002) Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. , J Am Coll Cardiol 39, 1061-5.
- 2.King T D, Thompson S L, Steiner C, Mills N L. (1976) Secundum atrial septal defect, nonoperative closure during cardiac catheterization. , JAMA 235, 2506-9.
- 3.Kim H H, Yi G J, Song S W. (2017) Late Migration of Amplatzer Septal Occluder Device to the Descending Thoracic Aorta. , Korean Journal of Thoracic and Cardiovascular Surgery 50, 47-49.
- 4.Divekar A, Gaamangwe T, Shaikh N, Raabe M, Ducas J. (2005) Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder.J Am Coll Cardiol,45:. 1213-1218.
- 5.Godart François, Houeijeh Ali, Recher Morgan, Marie Paule Guillaume, Domanski Olivia et al. (2015) 0355, Complications after transcatheter ASD closure with the amplatzer septal occluder. , Archives of Cardiovascular Diseases Supplements 7, 97.
- 6.M S Spence, S A Qureshi. (2005) . , Complications of transcatheter closure of atrial septal defects,Heart 91, 1512-1514.
- 7.Fischer G, Stieh J, Uebing A. (2003) Experience with transcatheter closure of secundum atrial septal defects using the Amplatzer septal occluder: a single centre study in 236 consecutive patients. , Heart 89, 199-204.
- 8.Chan K C, Godman M J, Walsh K. (1999) Transcatheter closure of atrial septal defect and interatrial communications with a new self expanding nitinol double disc device (Amplatzer septal occluder): multicentre UK experience. , Heart 82, 300-6.
- 9.Krumsdorf U, Ostermayer S, Billinger K. (2004) Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. , J Am Coll Cardiol 43, 302-9.
- 10.Amin Z, Hijazi Z M, Bass J L. (2004) Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. , Catheter Cardiovasc Interv 63, 496-502.
- 11.R K Nath, Pandit N. (2016) Asymptomatic late embolization of Amplatzer septal occluder device, Indian Heart J.
- 12.Abdelkarim E, EIH Mustapha, Abdennasser D, Drissi b. (2012) Silent embolization of an Amplatzer septal occluder into the left ventricular outflow tract requiring emergent surgical retrieval. , Ann Pediatr Cardiol 5(1), 89-91.
- 13.Ussia G P, Abella R, Pome G. (2007) Chronic embolization of an atrial septal occluder device: percutaneous or surgical retrieval? A case report. , J Cardiovasc Med 8(3), 197-200.
- 14.Errahmouni A, Hattaoui M E, Drighil A, Boumzebra D. (2012) Silent embolization of an Amplatzer septal occluder into the left ventricular outflow tract requiring emergent surgical retrieval. , Ann Pediatr Cardiol 5(1), 89-91.
