Excellent Completion Rate of 8-Weeks Hepatitis C Treatment in Prison; Results of French National Study.
Abstract
Rationale
Prisons are major reservoirs of hepatitis C virus (HCV) in which a therapeutic approach has been particularly difficult so far. Prevalence of viral hepatitis C (HCV) is higher in prison environment in France than in the general population and is estimated to be 4,8%. The impact in prison environment is little-known as based on local studies. Inmate health care falls under USMP (prison setting medical unit), hospital specific units as by the january 18, 1994 law. Access to antiviral c treatment for inmates has always been difficult in France, would it be for interferon and ribavirin or use of protease inhibitors, with less than 20% of treated patients. French recommendations for HCV screening recommend systematic screening of inmates. The arrival of all oral therapies by direct antiviral agents (DAA) with shorter treatment times was an opportunity for doctors to propose a treatment and the patient to accept it. In 2014, the French guidelines recommended that HCV carriers in prison should systematically be treated independently of the stage of fibrosis.
Objective of the Study PH8
Our objective was to evaluate the completion rate of an 8-week antiviral C treatment by sofosbuvir / ledipasvir regimen in non-cirrhotic genotype 1 patients in deprivation of liberty and achieve sustained virological response (SVR) and to measure the effectiveness of an 8-week treatment (by protocol analysis).
Methodology
prospective non-interventional multicenter trial among inmates with chronic hepatitis C genotype 1 with METAVIR fibrosis score F0 to F2 and who will receive a daily combination of sofosbuvir / ledipasvir for 8 weeks.
Results
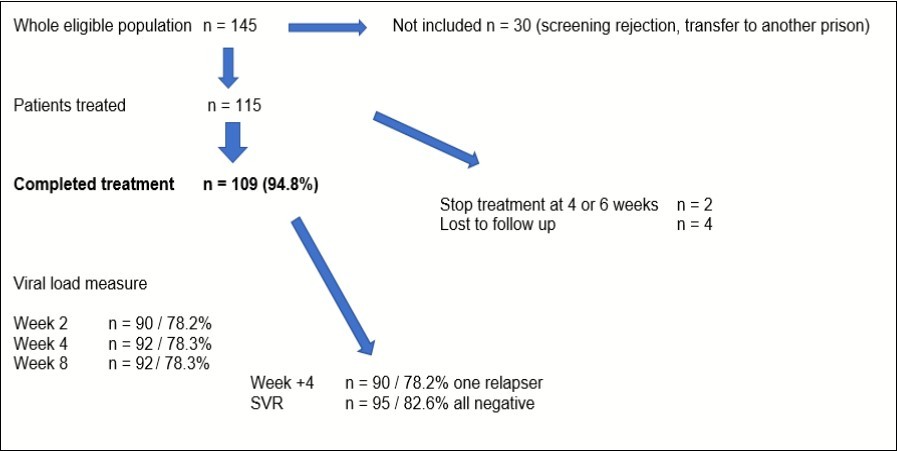
6 prison medical units included 115 consenting patients: there were 81% men, mean age 41 years (21 to 64 years). Route contamination was drug injection for 85%. HCV genotype was 1a for 74%, 1b for 24% and 2% none differenciated 1. Fibroscan mesure was available in 89 patients (mean score 3,5 KPa). Fibrotest was available in 37 patients with mean value 0.21. Eleven patients had Fibroscan and Fibrotest; 69% of patients were F0, 22% F1 and 9% F2. Average time between diagnosis and start of treatment was 3 weeks. We are sure that 109 patients (95%) completed DAA 8 weeks treatment; only 2 stopped DAA treatment before 8 weeks and 4 had no follow up after end of detention. HCV viral load was measured at W2 for 90 patients (78%), at W4 for 92 patients (78%), at end of treatment for 92 patients (78%), one month after treatment for 90 patients (78%) and 3 months after for 95 patients (93%). Only one viral load was positive, one month after treatment. Patient was retreated by sofosbuvir / velpastasvir. All HCV viral load 3 months after treatment negative; one patient took DAA only 6 weeks was cured.
Conclusions
In these study PH8, we observed completion rate of 94% for included patients in patient with 8 weeks ledipasvir/sofosbuvir regimen; data missed for only 4 patients and one relapsed. Short DAA treatment was efficient in prisoners and could be preferred in specific population.
Author Contributions
Academic Editor: Fatma mohammed Mady, Minia University Faculty of pharmacy, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2019 Jean-Marie RUIZ, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Hepatitis C virus (HCV) is a leading cause of liver-related morbidity and mortality worldwide, especially in France where prevalence rates range from 0,5 to 1% in the general population 1. Although highest European screening rate in France, 33% of patients didn’t take care of hepatitis C because there were no diagnosed; 75,000 people in France infected with HCV are without screening or treatment. French guidelines were to treat all patients even inmates and drug users, regardless the fibrosis level. Access of HCV screening, care and treatment in drugs users, prisoners and homeless was low in France. They were considered as difficult to treat populations. All these patients need a simplified pathway to reach HCV elimination. Prevalence of HCV was higher in prison environment than in the general population and is estimated by the PREVACAR study to be 4.8% 2. Systematic HCV screening on all new inmates has been recommended in France for the past 20 years. The impact in prison environment was little-known as based only on local studies. There were 168 different prisons in France with 66678 prisoners at the end of 2015. Inmate health care falls under USMP (prison setting medical unit), hospital specific units as by the January 18 1994 law, in the Ministry of health supervision. They were created after HIV epidemic in prisons during eighties. Nurses and doctors depend from publics hospitals. Principal HCV inmates studies are presented in Table 13, 4, 5, 6. Access to antiviral C treatment for inmates has always been difficult in France, would it be for interferon and ribavirin or the use of protease inhibitors, with less than 20% of treated patients. HCV prisoners have to be treated in French national guidelines and health ministry decision in June 2016. For many hepatologists, inmates had not be treated for multiple reasons: drugs users, too shirt delay of presence in prison, bad quality of follow-up. There were detention transfer, early releases and various duration of confinement. Also there was no minimal duration to treat. There no several somatic contraindications or language barrier for patients. In France, we had no cost limitations in practice to pay Direct Antiviral Agents (DAA) for inmates. The most important problem could be deadline between confinement and at the beginning of treatment and the speech held in / by the patient: "it is not the moment to treat hepatitis”. Also there were various levels of care according to MJU. The problem is exacerbated in prisons and other closed settings (i.e. jails, pre-trial detention centers, psychiatric institutions, etc.), where a high prevalence of risk behaviors, mainly injection drug use (IDU), is associated with a nearly ten times higher prevalence of HCV infection than that of the general population 2. Some intrinsic circumstances aggravate the problem, such as a substantial risk of HCV transmission during incarceration and after release, favored in part by an unawareness rate up to 25% 7, 8, 9, 10, 11, 12. Testing HCV in high-risk populations and subsequently treating the infected populations has been recommended for years as an epidemiological prevention measure to control a widespread infection, and also to prevent disease injury on an individual basis. However, there have been several classical barriers that have limited the universality of these policies]: lack of treatments with high beneficial/risk ratios and costs of treatments that influence on healthcare budgets of closed settings; penitentiary health care managed within each country by public agencies with different sensitivity to the health problem posed by prisons; persistence of indoor risk practices increase HCV transmission; limited capacity to access hospital-based hepatitis specialists; lack of specialist nurses; complex health care needs of prisoners; high detainee turnover, etc. The advent of powerful and safe direct acting antivirals in the last years, and the implementation of more rational penitentiary policies items of prevention and healthcare access must aid to face these challenges 13,14,15,16,17,18,19.
Table 1. State of art in France| year | author | % HCV | Specialized consultation | screening | Number of inmates | Rate of treated inmates |
| 2000 | REMY (3) | 6.7% | 10% | 36% | 52% | 3.9% |
| 2003 | REMY (4) | 6.9% | 22% | 64% | 49% | 13.9% |
| 2010PREVACAR | CHIRON(2) | 4.9% | 57% | 93% | 86% | 36% |
| 2012TRIPRI | REMY 5) | 4.5% | 49% | 78% | 59% | 29% |
| 2017BEH | REMY (6) | 4,3% | 56% | 70% | 39% | 19% |
Arrival of all oral therapies by DAA with shorter treatment times was an opportunity for doctors to propose a treatment and the patient to accept it... Main objective of this study was to evaluate the completion rate of an 8-week antiviral C treatment by sofosbuvir / ledipasvir regimen in non-cirrhotic genotype 1 patient’s prisoners and achieve sustained virological response (SVR) and to measure the effectiveness of an 8-week treatment by protocol analysis.
Methods
Study Design
This was an open-label, single-arm, phase-IV clinical trial of low-grade of intervention. It was carried out between July 2016 and June 2017 in 6 French penitentiary centers. Age higher than 18, HCV genotype 1, lack of cirrhosis and acceptance to participate were eligibility criteria. At baseline, variables including anthropometrics, demographics, risk behaviors, medical history, particularly if related to HCV infection and psychiatric conditions, were recorded. A serum sample that included a complete blood cell count, biochemistry and viral markers (HBs Ag, HBs and HBc antibodies, HIV antibody) was also obtained. HCV infection characterization (i.e. viremia and genotype, clinical and pharmacological history, ultrasonography and FibroScan® -Echosens™), and the subsequent systematic treatment of viremic patients were performed. French recommendations were to used FibroScan® or Fibrotest® as equal to evaluate liver fibrosis before HCV treatment. In addition, a strategy of follow-up was planned for those treated patients who left prison, either because they were transferred to another prison, or because they reached freedom. Every chronic infected patient whose stay in prison was expected to be longer than 30 days was offered treatment in accordance with national guideline to treat every patient with the simplest antiviral regimen based on a fixed-dose combination tablet containing 400mg of sofosbuvir and 90mg of ledipasvir (Gilead Sciences, Inc., Foster City, CA 94404), administered orally once daily during 8 weeks without ribavirin. This combination was selected according to the French guidelines and taking into account the specific availability of antiviral drugs. Therefore, the industry financial support in the study did not influence in any way the decision to treat patients with this single DAA combination. Treatments were prescribed by hepatologist and 8 weeks according to the characteristics of the disease. The therapy was being administered under direct observation supervised by the staff from health unit. During the periods in which detainees were out of prison, a counting pill and a direct interview were established for assessing compliance. Request administrative permissions and informed consent were obtained before starting study.
Efficacy Assessments.
Plasma HCV-RNA levels were measured with the use of the COBAS TaqMan HCV-RNA assay, version 2.0 (Roche Molecular Systems, Pleasanton, CA), with a lower limit of quantification of 25 IU per milliliter and a lower limit of detection of 10 IU per milliliter. HCV-RNA levels were measured at baseline, at weeks 4, 8, 12, and at the follow-up visit held 12 weeks after EOT. A sustained virological response (SVR) was defined as a viral load <10 IU per milliliter 3 months after EOT.
Ethics
The study was approved by all Institutional Review Boards and by the Ethics Committee of and was conducted in compliance with the Declaration of Helsinki and International Conference on Harmonization guidelines. Throughout the study prisoners were free to question the investigators and to refuse to participate without any repercussions during the incarceration period. All patients included received a written informed consent provided before enrollment.
Statistical Analysis
A descriptive analysis was performed. Categorical variables were described with percentages, and continuous variables were described with mean and standard deviation or median and range/interquartile range (IQR) as appropriate. A 95% confidence interval (CI) was used to estimate proportions. The statistical analysis was performed with SPSS Statistics for Windows, Version 21.0 (IBM Corp, Chicago, Armonk, NY, USA). All -values were two-tailed. Statistical significance was defined as < 0.05.
Results
Characteristics of the Study Population
Six prison medical units included 115 consenting patients: there were 81% men, mean age 41 years (21 to 64 years). Route contamination was drug injection for 85%, others drug uses for 9% and unknown for 5%; 41% were treated by burprenorphin and 32% by methadon. HCV genotype was 1a for 74%, 1b for 24% and 2% none differentiated 1. Mean Initial HCV viral load was 1875389 UI (609 to 25 000 000).
Fibrosis Stage Distribution (F)
Fibroscan® measure was done in 89 patients (mean score 3,5 KPa). Fibrotest® was done in 37 patients with mean value 0.21. Eleven patients had Fibroscan® and Fibrotest®. Sixty nine per cent of patients were classified without fibrosis (F0), 22% with low fibrosis (F1) and 9% mild fibrosis (F2).
HCV Treatment
Average time between diagnosis and start of treatment was 3 weeks. We are sure that 109 patients (95%) completed DAA 8 weeks treatment; only 2 stopped DAA treatment before 8 weeks and 4 had no follow up after end of detention. HCV viral load was measured at W2 for 90 patients (78%), at W4 for 92 patients (78%), at end of treatment for 89 patients (77%), one month after treatment for 82 patients (71%) and 3 months after for 90 patients (78%). Only one viral load was positive, one month after treatment. Patient was retreated by sofosbuvir / velpastasvir. All HCV viral load 3 months after treatment negative; one patient took DAA only 6 weeks was cured. The main objective of study has been achieved with rate compliance of 94.8%. Follow up of HCV treated patients was detailed on Figure 1.
Discussion
Although the management of penitentiary healthcare has improved considerably throughout recent years, it still faces barriers that limit the goal of achieving an equitable and non-discriminative health care access for inmates. The implementation in prisons of a program of eradication of HCV based on interferon-free DAA-based regimens searches the former goal and also adds to the benefit of treating addictions and the intrinsic role of social rehabilitation of prisons. Thus, imprisonment can provide a unique opportunity to improve the health of these individuals. In addition to the beneficial effects at an individual level, HCV elimination in prisons would generate relevant benefits for public healthcare. Indeed, it is well known the persistence of high-risk behaviors both indoor and after release from incarceration (especially among people with substance use disorders), favoring HCV infection and transmission inside and outside prison.
Conclusion
In these study PH8, we observed excellent completion rate for included patients with 8 weeks ledipasvir/sofosbuvir regimen. Short DAA treatment was efficient in prisoners and could be preferred in this specific population. So we could confirm that hhepatitis care in prison is possible. Treatment for HCV in prisons is feasible and successful but depends on local medical care and there were many differences in different centers. In fact, percentage of treated patients could be potential higher than outside patients with short HCCV pathway. Hepatology consultation in jailhouses is very important to create link between inside and outside care. Jailhouses could be an ideal place of treatment but it needs better access to FIBROSCAN® or FIBROTEST® and more hepatology inside consultation. Study highlights are current known. Prisons are major reservoirs of HCV. There is a substantial risk of HCV transmission during incarceration. HCV treatment in prisons faces different challenges that so far have prevented its universal implementation. A sustained and universal “test and treat” strategy in a prison led to the eradication of HCV. It also prevented new incident cases of HCV (i.e. intra-prison transmission) Eradication of HCV in prisons are feasible and beneficial. Spreading this strategy should entail a public health impact
Disclosures
This study had an institutional support from GILEAD laboratory who did not directly or indirectly participate in the collection, data analysis nor redaction of this survey.
References
- 1.Pioche C, Pelat C, Larsen C, Desenclos J C, Jauffret-Roustide M et al. (2016) Estimation of hepatitis c prevalence in the general population, metropolitan France. , Bull Epidemiol Hebd 13(14), 224-229.
- 2.Chiron E, Jauffret-Roustide M, Y Le Strat, Chemlal K, Valantin M A et al. (2013) . Prevalence of HIV and hepatitis C virus among French prison inmates in 2010: Results from Prevacar survey 2010. Bull Epidemiol Hebd 35(36), 445-50.
- 3.Remy A J, Benhaïm S, Khemissa F. (2003) Care of HCV patients in prison. , Revue du Praticien 17, 1325-1327.
- 4.Remy A J. (2006) et les UCSA de France. Increase of screening and treatment of HCV patients in prison : comparison 2000-2003. Presse Médicale. 35(9), 1249-1254.
- 5.Remy A J, UCSA de France. (2014) Treatment of the hepatitis C in prison in France in 2011-2012: more patients treated in fewer medical jailhouse units : results of national practice survey. UEGW Congress. , Vienne
- 6.Remy A J, Canva V, Chaffraix F, Hadey C, Harcouet L et al. (2017) Hepatitis C in prison settings in France: a national survey of practices for. , Bull Epidémiol Hebd 14(15), 277-84.
- 7.Larney S, Kopinski H, Beckwith C G. (2013) Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology.58: 1215-24.
- 8.Dolan K, Wirtz A L, Moazen B. (2016) Global burden of HIV, viral hepatitis, and tuberculosis in prisoners and detainees. , Lancet 388, 1089-102.
- 9.Cepeda J A, Niccolai L M, Lyubimova A. (2015) High-risk behaviors after release from incarceration among people who inject drugs in St. , Petersburg, Russia, Drug Alcohol Depend 147, 196-202.
- 10.Remy A J. (2007) Why treat HCV patients in prison ?. , Gastroentérologie Clinique et Biologique 31, 566-8.
- 11.Remy A J. (2016) Incidence of the hepatitis C in prison in France: results of a study by POCT. , J of Liver 5(2), 33.
- 12.Larney S, Kopinski H, Beckwith C. (2013) Incidence and prevalence of hepatitis C in prisons and other closed settings : results of a systematic review and meta-analysis. Hepatology. 58, 1215-23.
- 13.Boonwaat L, Haber P S, Levy M H. (2010) Establishment of a successful assessment and treatment service for Australian prison inmates with chronic hepatitis C. , Med J Aust 192, 496-500.
- 14.Allen S A, Spaulding A C, Osei A M. (2003) Treatment of chronic hepatitis C in a state correctional facility. , Ann Intern Med.; 138, 187-90.
- 15.He T, Li K, Roberts M S. (2016) Prevention of Hepatitis C by screening and treatment in U.S. prisons. , Ann Intern Med 164, 84-92.
- 16.Martin N K, Vickerman P, Brew I F. (2016) Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. , Hepatology 63, 1796-808.
- 17.Liu S, Watcha D, Holodniy M. (2014) Sofosbuvir-based treatment regimens for chronic, genotype 1 hepatitis C virus infection in U.S. incarcerated populations: a cost-effectiveness analysis. , Ann Intern Med 161, 546-53.
- 18. (2017) Cunningham EB,HajarizadehB,BretanaNA,Amin J,Betz-StableinB,Dore GJ,LucianiF,TeutschS,Dolan K,Lloyd AR,GrebelyJ.HITS-p investigators. Ongoing incident hepatitis C virus infection among people with a history of injecting drug use in an Australian prison setting, 2005-2014: The HITS-p study. J Viral Hepat. 24(9), 733-741.