Tridax Procumbens Inhibitory Properties of Alpha Amylase
Abstract
The purpose of this study was to investigate the effects of extracts of Tridax procumbens leaves a traditional medicinal plant, on α-amylase activities in vitro. The air-dried aerial parts of Tridax procumbens leaves were extracted with Petroleum ether and Ethanol. The results of both enzyme inhibition activities were found in a dose-dependent manner. The strongest activity of α-amylase inhibition was found in Petroleum ether extract (IC50 = 10.436 mg/mL) followed by ethanol extract (IC50 = 12.65 mg/mL) compared with acarbose having an IC50 value of 0.044 mg/mL. All extracts from this plant possess potent α-amylase activity inhibition which may offer a better therapeutic strategy to minimize postprandial hyperglycemia and its complications.
Author Contributions
Academic Editor: Loai Aljerf, Department of Life Sciences, Faculty of Dentistry, Damascus University
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2021 Kwesi Akonu Adom Mensah, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Background
Diabetes mellitus is a chronic endocrine disorder caused by an absolute or relative lack of insulin, which affects the metabolism of carbohydrates, proteins, fat, electrolytes and water. It includes a group of metabolic diseases characterized by hyperglycemia in post prandial and/or fasting state, in which blood sugar levels are elevated either because the pancreas does not produce enough insulin or cells do not respond to the produced insulin 6. In its severe form, it is accompanied by ketosis and protein wasting. Diabetes leads to a state in which the homeostasis of carbohydrates and lipid metabolism is improperly regulated by the pancreatic hormone, insulin ultimately resulting in increased blood glucose level. 14. Diabetes mellitus is one of the challenging diseases in the field of the medicinal chemistry.
Diabetes is the world’s largest endocrine disorder 14. Therefore, there is the need for a therapeutic approach to treat diabetes. The most common method is to decrease postprandial hyperglycemia 9. This can be achieved by the inhibition of carbohydrate hydrolyzing enzymes like alpha amylase and alpha glucosidase 14. Alpha glucosidase and alpha amylase are the important enzymes involved in the digestion of carbohydrates. Alpha Amylase is involved in the breakdown of long chain carbohydrates and alpha glucosidase breaks down starch and disaccharides to glucose. They serve as the major digestive enzymes and help in intestinal absorption. Alpha amylase and glucosidase inhibitors are the potential targets in the development of lead compounds for the treatment of diabetes 8.
Natural products from plants have been used for the treatment of diabetes, mainly in developing countries like Ghana where the resources are limited and affordability and access to modern treatment is a problem. Use of natural products has existed for a long time without any proper research conducted in the country into their efficacies and side effects. Extensive research has been carried out elsewhere to screen the bioactivity of these natural products because of their significant importance in health care and medicine. Plant food rich in secondary metabolites have been reported to cause effects similar to insulin in the utilization of glucose and act as good inhibitors of key enzymes like alpha amylase and alpha glucosidase associated with type 2 diabetes and lipid peroxidation in tissues. Studies have also shown that the bioactivity of secondary metabolites in plants is linked to their antioxidant activity and many of these plants also possess hypoglycemic properties 23. To prevent the effects of free radicals, antioxidants donate electrons to rampaging free radicals and neutralize them, thereby reducing their capacity to damage tissue 24.
Higher plants, animals and microorganisms are found to produce a large number of different protein inhibitors of alpha amylases and alpha glucosidases in order to regulate the activity of these enzymes 3. Some of these enzyme inhibitors act by directly blocking the active center of the enzyme at various local sites3. In animals, alpha amylase inhibitors decrease the high glucose levels that can occur after a meal by slowing the speed with which alpha amylase can convert starch to simple sugars 20. This is of importance in diabetic individuals since low insulin levels prevent the fast clearing of extracellular glucose from the blood 20. Hence, diabetics tend to have low alpha amylase levels in order to keep their glucose levels under control. These inhibitors alter the digestive action of alpha amylases and proteinases in the gut of insects and inhibit their normal feeding behavior. Thus, alpha amylase inhibitors have potential roles in controlling blood sugar levels and crop protection 20. Alpha-amylase can be found in various organisms and show diverse substrate specificities while possessing a common topology formed from three domain, one of which being a typical alpha-beta barrel. Inhibition of insect’s alpha amylase is a proposed method of crop protection. On the other hand, inhibition of mammalian alpha-amylase is a proven therapeutic approach in diabetes and related disorders 22. Therefore, this research seeks to determine the ability of Tridax procumbens to inhibit the activity of alpha amylase and help in treatment of diabetes mellitus.
Tridax procumbens is a plant belonging to the daisy family, and found in various tropical and subtropical regions as well as mildly temperate regions worldwide 20. It habitats waste places, road sides and hedges throughout Ghana 2. The herb is used locally in Ghana for the treatment of wounds which lead to bleeding and also used to cause wounds to heal faster. It is boiled by some indigenes and drank as tonic for treatment of several ailments such as diabetes, typhoid fever and malaria. The plant is administered mainly by boiled extracts, macerated extract or raw squeezed extract.
Problem Statement
Diabetes Mellitus is the world’s largest endocrine disease affecting millions of people. Individuals in developing countries are unable to afford conventional drugs to manage the condition. Hence, this work is aimed at studying the ability of Tridaxprocumbensto inhibit alpha amylase.
Hypothesis
Plant extracts of Tridax procumbens decrease the activity of alpha amylase.
Main Objective
To investigate the inhibitory effects of Tridax procumbens extracts on the activity of alpha amylase.
Specific Objectives
To determine the effect of Petroleum ether extracts of Tridaxprocumbenson alpha amylase activity.
To determine the effect ethanolic extract of Tridaxprocumbens on alpha amylase activity.
To project the efficacy of Tridaxprocumbens in managing the diabetes mellitus disorder.
Literature Review
Alpha Amylase
Alpha-amylase ( EC 3.2.1.1, PDB ID: 5U3A) is an enzyme that acts upon large linear carbohydrate (Starch) polymers at internal bonds. The hydrolytic products have alpha- configuration. In nature, starch (Carbohydrate) is the most abundant polysaccharide food store after cellulose and the primary accessible source of carbon and energy on the Earth. It is synthesized by plants and utilized in food, textile, paper, alcohol, pharmaceutical industries. Starch is deposited in plant cells in the form of granules as reserve material. 7
The enzyme is a glycoprotein 7. Its single polypeptide chain of about 475 residues has two free thiol groups, four disulfide bridges, and contains a tightly bound Ca2+, 27 (see Appendices A, B and C). It exists in two forms (PPAI and PPAII), which have identical enzymatic properties and molecular mass of 55.4 kDa, differing only in electrophoretic mobility and Pi 5, 7
The structures of both forms of PPA consist of a major domain A (residues 1-99 and 170- 404) organized as an (alpha/beta)8-barrel that contains a loop (domain B). Following the C- terminal domain C (residues 405-496) is a ten-beta-stranded Greek key motif. 10
Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine. α -amylase and α-glucosidase are key enzymes involved in carbohydrates breakdown and intestinal absorption, respectively. It has an optimum pH of 7.0. 29 Inhibition of these enzymes hamper blood glucose level increase after a carbohydrate diet and can be an important strategy in the management of non-insulin-dependent diabetes mellitus (NIDDM). In general, there is very little biological knowledge on the specific modes of action in the treatment of diabetes but most of the plants have been found to contain secondary plant metabolites like glycosides, alkaloids, terpenoids, flavonoids etc., that are frequently implicated as having antidiabetic effects by inhibiting the activity of alpha amylase.
Due to the role alpha amylases play in the metabolism of carbohydrates, research has been conducted to analyze the benefits of modulating and regulating the activities of this enzyme. Most of these researches have been done through inhibition and activation properties of various compounds. According to research carried out, inhibitors for alpha amylase were determined to be:
Phenolic compounds 11
Urea and other amide reagents 14.
Diabetes Mellitus
Diabetes mellitus is one of the challenging diseases in the field of the medicinal chemistry.
It is classified in to two types 19:
Type 1- Insulin dependent diabetes mellitus. Type 2- Non-insulin dependent diabetes mellitus.
Type 1 diabetes (T1D) is a chronic disease caused by immune-mediated destruction of insulin producing beta cells in the pancreas. The destruction of beta cells results in insulin insufficiency, and patients develop life-threatening hyperglycemia that clinically manifests with weight loss, polyuria, and polydipsia28. These are due to defective transport of glucose from the bloodstream into tissues, resulting in increased glucose levels in the blood, elevated glucose in the urine, and concomitant calorie and fluid losses in the urine. When insulin levels fall to such low levels that lipolysis cannot be suppressed, products of fat metabolism called ketone bodies (primarily acetoacetate and β- hydroxybutyrate) accumulate in the blood, leading to metabolic acidosis and compensatory respiratory alkalosis due to hyperventilation. If untreated, compensatory mechanisms eventually fail and ketoacidosis results in cerebral edema, mental confusion, unconsciousness, coma, and death15. Data from various researchers indicates that the disease process may be delayed by administering oral insulin to induce insulin specific regulatory T-cells in the gut, resulting in decreased inflammation in the pancreas.
Research has shown that Type 2 DM (T2DM) accounts for majority (90-95%) of diabetes and poses a huge burden on healthcare systems especially in developing countries 20. Type 2 Diabetes occurs when the pancreatic beta cells cannot produce enough insulin or the cells acquire some degree of resistance to insulin. Normal regulation of glucose metabolism is determined by a feedback loop involving the islet β-cell and insulin-sensitive tissues in which tissue sensitivity to insulin determines the magnitude of the β-cell response. When insulin resistance is present, the β-cell maintains normal glucose tolerance by increasing insulin output. It is only when the β-cell is incapable of releasing sufficient insulin in the presence of insulin resistance that glucose levels rise 16.
Current Treatment
Conventional therapies, as of yet, have been unable to achieve a cure for diabetes mellitus. Hence, systematic and intensive search in medicinal plants for new drugs to treat diabetes mellitus especially type 2 seem to be of great utility. Acarbose, currently marketed as a medicine in the treatment of diabetes, lowers post-prandial peaks of glucose. However, acarbose is principally known as an alpha-glucosidase inhibitor and causes side effects such as, abdominal distension, flatulence, meteorism and possibly diarrhea. Therefore it is attractive to find a substance that has strong inhibitory activity against α-glucosidase but minor effect on α-amylase activity 19.
Acarbose inhibits enzymes (glycoside hydrolases) needed to digest carbohydrates, specifically, alpha-glucosidase enzymes in the brush border of the small intestines and pancreatic alpha-amylase. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine, whereas the membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the small intestine. Inhibition of these enzyme systems reduces the rate of digestion of complex carbohydrates. Less glucose is absorbed because the carbohydrates are not broken down into glucose molecule 19.
Herbal Medicine
Many common herbs and spices are claimed to have blood sugar lowering properties that make them useful for people with or at high risk of type 2 diabetes.
A number of clinical studies have been carried out in recent years that show potential links between herbal therapies and improved blood glucose control, which has led to an increase in people with diabetes using these more 'natural' ingredients to help manage their condition. 12
Herbal medicine in the scientific community is questioned on the basis of their modes of action and the ability of some active ingredients contained in them to interact with diabetic drugs and produce undesirable effects. Many researchers have therefore committed to demystifying herbal medicine. In the article “Antidiabetic effect of oral borapetol B compound, isolated from the plant Tinosporacrispa, by stimulating insulin release,” F. E. Lokman et al., have presented an evaluation of the antidiabetic property of a biologically active small compound borapetol B (C1) isolated from T. crispain normoglycemic control using Wistar (W) and spontaneously type 2 diabetic Goto-Kakizaki (GK) rats. They found that an acute oral administration of the compound significantly improves blood glucose levels in the treated group in comparison to the placebo group. They observed that plasma insulin levels were significantly enhanced by 2-fold in treated W and GK rats compared to the placebo group at 30 minutes. Their study provides evidence that borapetol B (C1)’s antidiabetic property is mainly due to stimulation of insulin release. 6
Another source of contention involves the mode of extraction of plant Extracts for use in diabetes management. It should be noted that plant extraction with a particular solvent is dependent on the type of plant and plant part. This is seen in work done by 2. where efficacy of extract depended on the type of plant and solvent used for extraction.
Tridax Procumbens
The plant researched in this study, is Listed as a weed and a pest plant, it has been known by several names including ‘Tridax daisy’ in English, and ‘Herbecaille’ in French (see Appendix D). Many reports have focused on the immense potential of this plant which has antimicrobial, wound healing, anti-inflammatory and immunomodulatory properties (P et al., 2011). Antioxidant properties have also been demonstrated in various researches 21. Antioxidants can scavenge free radicals and play important role in prevention of diabetes. The role of oxidative stress in diabetes and diabetic complications has been reported by many researchers. 25
Previous Studies on Tridax Procumbens
Work by 31 determined by in-vitro assay that the Tridax procumbens plant extracts had alpha amylase inhibitory activities and these activities were dependent on the type of solvent used in extraction of Tridaxprocumbens. Among the extracts, methanol exhibited highest α-amylase activity. The methanol extract of Tridaxprocumbens exhibited highest α-amylase with an IC50 value of >10 µg/mL. The petroleum ether extract of Tridaxprocumbensextract showed α- amylase inhibitory activity with IC50 value of 70 µg/mL. 20, also showed that the active components found in the extract include alkaloids, flavonoids, glycosides, saponins, polyphenols, tannins. Their research determined that inhibitory activities could be as a result of presence of these bioactive compounds and their interaction with proteins. The mode of inhibition of the aqueous extract of Tridaxprocumbensleaf on alpha-amylase was determined using the Lineweaver-Burk plot in this study, which displayed competitive inhibition of the enzyme.
Materials and Methods
Isolation of Alpha Amylase from Malted Maize
This was done according to Bendelow’s method with some modifications. Maize obtained from the laboratory was subjected to the malting process to allow for a short duration of germination where alpha amylase is activated to break down stored carbohydrates in the seeds in order to nourish and support the growing young plant. After a considerable malting of the maize it was subjected to milling into a uniform powder. A mass of 3 g of milled malt sample was weighed into centrifuge tubes and 12 mL of phosphate buffer at pH of 6.9 (pKa 7.21). The enzymes were allowed to extract into the extraction medium for 30 minutes after which the enzyme suspension was centrifuged at a speed of 4500 rpm for 15 minutes using a swinging bucket rotor type centrifuge. The supernatant obtained was decanted and according to research by 32. contained alpha amylase of unknown activity. The pH of the supernatant obtained was reduced to 5.5 by the addition of 0.2 M hydrochloric acid. After this, there was a confirmatory test to determine the presence of the alpha amylase. To achieve this, 1mL of the enzyme extract was added to 5 mL of 1% starch solution and then 1 mL of iodine solution was added. The fading of the blue-black color with time is an indication of the presence of alpha amylase.
Crude Enzyme Concentration Determination
This was done according to Duffus’s method with some slight modifications. Different concentrations of crude alpha amylase were prepared. (1:10,1:100 and undiluted). Biuret reagent of volume 2 mL was then added to each of the concentrations and absorbance was taking at 562 nm wavelength using a UV/VIS Spectrophotometer.
Enzyme Activity (Control)
This was done according to Sindhu’s method with some modifications. Alpha amylase was incubated with 250 ul alpha amylase for 10 minutes at 25˚C. To the incubated amylase, 500 ul of distilled water was added and 250 ul of starch solution of concentration 1% was added. The resulting solution was incubated for 3 minutes at 25˚C. Acidified Iodine solution of volume 500 ul was added to stop the reaction. Absorbance was taken at 620 nm wavelength using a UV/VIS spectrophotometer.
Sample Collection
Tridax procumbens leaves were obtained from the lawn in front of the department of biochemistry after assessment was made on impact of human and other mechanical activities on the normal growth of the plant. Upon harvesting of the leaves, they were sent to the Teaching Assistant for verification of authenticity.
Sample Treatment
Fresh green leaves of the Tridax procumbens were washed with distilled water 3 times and air dried for 2 days. The dried leaves were subjected to blending using a warring blender (Speed 4, 10 Watts) for 15 minutes until a fine powder was obtained. A mass of 15 grams of the powdered Tridax procumbens according to Adrian’s method with slight modifications, was weighed into a beaker and 250 mL of Ethanol was added. Another 15 grams mass of powdered Tridaxprocumbenswas weighed into another beaker and 250 mL of petroleum ether was added. The mixtures were then transferred into 2 clean clearly labeled volumetric flasks. The mixtures were left standing with intermittent shaking for 3 days. Extract for each solvent was then obtained by filtration and evaporation of the sample. The slurry obtained was then stored for further analysis to be carried on.
Preparation of Reagents
Preparation of Biuret Reagent
This was done according to Mulimami’s method. A volume of 50 mL of 0.2 M NaOH was taken into two separate beakers respectively and labelled A and B. From Beaker A, 25 mL of NaOH was taken to dissolve 0.9 g of NaKTatrate. Another 10 mL was taken from the same beaker and used to dissolve 0.3 g of CuSO4. The two solutions prepared were mixed and the remaining contents of beaker A was added. To the obtained solution 0.5 g of KI was added to dissolve well. The contents of beaker B was then added to the resulting solution.
Preparation of Iodine Solution
This was done according to the method described by 30. Iodine crystals of mass 0.02 g was taken together with 0.07 g of KI using an electronic balance. The KI was dissolved in 2.50 mL of distilled water and the iodine crystals were added. The solution was made up to 25 mL mark with distilled water.
Preparation of Standard Curves
Preparation of BSA Standard Curve
This was done according to the method described by 30. A mass of 0.015 g of BSA was weighed using electronic balance (Explorer® Analytical EX324N/AD, Linearity ± 0.0002 g, Maximum Capacity 320 g) and dissolved in 3 mL of water. Varying concentrations of BSA solution was prepared (50 ,100 ,250 ,500, 750 mg/mL) and the final volume of each of the concentrations was topped up to 1 mL. To each of the concentrations, 3 mL of the biuret reagent was added and incubated for 10 minutes after which absorbance was taken at 562 nm wavelength using a UV/VIS Spectrophotometer.
Preparation of Starch Standard Curve
This was done according to the method described by P et al., 2011. A mass of 0.085 g of 100% starch was weighed and dissolved in 8.5 mL of water and heated gently. Varying concentrations of the above stock was prepared. (1, 2, 4, 8, 10, 12,16,10 mg/mL). Two drops of iodine solution were added to each of the prepared starch concentrations and absorbance was taking at 620 nm wavelength using a UV/VIS Spectrophotometer.
Phytochemical Analysis of Tridax Procumbens Leaves.
This was a qualitative test performed to ascertain the kinds of phytochemicals present in Tridaxprocumbens(seeAppendixE). Phytochemical compositions of the leaves were determined using the methods variously described by Gramer and Walker with slight modification (Gramer & Walker, 2007).
Test for Alkaloids (Wagner’s Reagent)
Wagner’s reagent was prepared by dissolving 0.04 g of iodine crystals in 2 mL of water. A volume of 1 mL of the extract was aliquoted and 3 drops of the Wagner’s reagent was added to it. Observation for reddish brown was expected to indicate the presence of alkaloids.
Test for Flavonoids
From the plant extract, 1 mL was aliquoted and 3 drops of 20% lead acetate (0.2 g of lead in 1 mL of water). Yellow precipitate was expected to indicate the presence of flavonoids.
Test for Phenols
A volume of 1 mL of the extract was picked and 1 mL of water was added to it after which 1mL of 50% ferric chloride (0.5 g in 1 mL of water). Blue color was expected to indicate the presence of phenols.
Test for Protein (Biuret Method)
From the extract, 2 mL was picked and 0.5 mL of biuret reagent was added. It was then allowed to stand for 5 minutes and the color change was observed and recorded.
Test for Tannins
To 1mL of the extract, 1 mL of 5% ferric chloride was picked and added to 1 mL of 90% ethanol. This solution was then added to 1 mL of the extract. The colour change was observed and recorded.
Test for Saponins
A volume of 1 mL of the extract was picked and 0.5 mL of water was added. Upon shaking, a persistent froth was formed it was then mixed with three drops of olive oil. The presence of emulsion was an indication of the presence of saponins.
Test for Glycosides (Bontrager’s Test)
A volume of 1 mL of the extract was picked and boiled with 1 mL of 10% HCl in a water bath for five minutes, then 1 mL of chloroform was added and the observation of colour change was made and recorded.
Test for Terpenoids
To 1 mL of the extract, 1 mL of chloroform was added after which 2 drops of concentrated tetraoxosulphate (iv) acid (H2SO4) was added and kept for 60 seconds. Appearance of yellow colour in the lower layer was expected as an indication of the presence of terpenoids
Test for Steroids
To 1 mL of the extract ,1 mL of chloroform was added and 5 drops of concentrated hydrochloric acid (HCl) was added and kept for 60 seconds. Appearance of red colour in the lower layer was expected as an indication of the presence of steroids.
Inhibition Assay
Percentage Inhibition
This was done according to the method described by 31. Varying concentrations of both ethanolic and methanolic extract of the Tridaxprocumbens(30,40,50,60,70,80 mg/mL) were prepared Out of each of the prepared concentrations, 250 µl was picked and incubated with 250 µl of alpha amylase in test tubes for 10minutes at room temperature. After, 500 µl of distilled water was then 250 µl of 1% starch solution. was also added and incubated for 3 minutes. After the three minutes, 500 µl of acidified iodine was added to stop the reaction and absorbance was taking at 620 nm (see Appendix F and G). A blank and a control were also prepared. The control did not contain the inhibitor. The percentage inhibition was calculated according to the formula; Inhibition (%) = Abs 620 (control) – Abs 620 (extract) x 100
Abs 620 (control)
Test for Steroids
To 1 mL of the extract ,1 mL of chloroform was added and 5 drops of concentrated hydrochloric acid (HCl) was added and kept for 60 seconds. Appearance of red colour in the lower layer was expected as an indication of the presence of steroids.
Inhibition Assay
Percentage Inhibition
This was done according to the method described by 31. Varying concentrations of both ethanolic and methanolic extract of the Tridaxprocumbens(30,40,50,60,70,80 mg/mL) were prepared Out of each of the prepared concentrations, 250 µl was picked and incubated with 250 µl of alpha amylase in test tubes for 10minutes at room temperature. After, 500 µl of distilled water was then 250 µl of 1% starch solution. was also added and incubated for 3 minutes. After the three minutes, 500 µl of acidified iodine was added to stop the reaction and absorbance was taking at 620 nm (see Appendix F and G). A blank and a control were also prepared. The control did not contain the inhibitor. The percentage inhibition was calculated according to the formula; Inhibition (%) = Abs 620 (control) – Abs 620 (extract) x 100
Abs 620 (control)
Mode of Inhibition
The mode of inhibition of α -amylase by the leaf extract was conducted using the extract with the lowest IC50 according to the modified method described by 32. Briefly, 250 µL of the extract (5 mg/mL) was preincubated with 250 µL of α -amylase solution for 10 min at 25°C in one set of tubes. In another set of tubes α -amylase was preincubated with 250 µL of phosphate buffer (pH 6.9). 250 µL of starch solution at increasing concentrations (0.30–5.0 mg/mL) was added to both sets of reaction mixtures to start the reaction. The mixture was then incubated for 10 min at 25°C and 500 µl of acidified iodine was added to stop the reaction and absorbance was taken at 620 nm. The amount of starch remaining was determined spectrophotometrically using a Starch standard curve and converted to reaction velocities. A double reciprocal plot (1/V versus 1/(S)) where V is reaction velocity and (S) is substrate concentration was plotted.The type (mode) of inhibition of the crude extract on α-amylase activity was determined by analysis of the double reciprocal (Lineweaver-Burk) plot using Michaelis- Menten kinetics.
Data Analysis
Data obtained from the experiment were analyzed statistically using Excel 2016. Analysis tool pack was used in Data projections and graphing.
Results
Different extracts were obtained from Tridax procumbens leaves from the different solvents (ethanol and Petroleum ether) employed. Petroleum ether extract has the highest percentage yield of 9%, followed by Ethanol (7.30%). The phytochemical composition of the Tridax procumbensextracts of ethanol and Petroleum ether indicated the presence of flavonoids, tannins, and reducing sugar among others. Alpha-amylase inhibition potential of the Tridax procumbens extracts was determined.
Extrapolation of α-amylase effectiveness from the dose response curve showed that Petroleum ether extract contained the most potent α -amylase inhibitor with an IC50 value of 10.436 mg/mL.
The mode of inhibition of the aqueous extract of Tridax procumbens leaf on α-amylase activity was determined using the Lineweaver-Burk plot which showed that the extract displayed a non- competitive inhibition of the enzyme activity. Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10. Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9
Table 1. Concentrations and corresponding absorbance of BSA used for enzyme protein estimation| Concentration, g/mL | Absorbance 562nm |
| 0.00 | 0.015 |
| 0.05 | 0.074 |
| 0.10 | 0.101 |
| 0.25 | 0.116 |
| 0.50 | 0.136 |
| 0.75 | 0.165 |
| Concentration, g/mL | Absorbance 620nm |
| 1 | 0.228 |
| 2 | 0.276 |
| 4 | 0.348 |
| 8 | 0.390 |
| 10 | 0.410 |
| 12 | 0.436 |
| 16 | 0.460 |
| 20 | 0.472 |
| Concentration | Absorbance 562nm |
| Blank | 0.028 |
| 1:100 | 0.065 |
| 1:10 | 0.081 |
| Undiluted | 0.102 |
| Concentration, g/mL | Absorbance 562nm |
| 4 | 0.294 |
| 8 | 0.321 |
| 12 | 0.332 |
| 16 | 0.341 |
| 20 | 0.397 |
| Concentration, g/mL | Absorbance 562nm |
| 4 | 0.311 |
| 8 | 0.359 |
| 12 | 0.367 |
| 16 | 0.373 |
| 20 | 0.384 |
| Concentration, g/mL | Absorbance 562nm |
| 4 | 0.327 |
| 8 | 0.377 |
| 12 | 0.402 |
| 16 | 0.421 |
| 20 | 0.448 |
| Concentration of inhibitor, mg/mL | % Inhibition by Petroleum ether extract | % Inhibition by Ethanol extract |
| 4 | 30.4 | 24.9 |
| 8 | 45.1 | 37.8 |
| 12 | 56.9 | 53.6 |
| 16 | 63.6 | 61.5 |
| 20 | 75.1 | 64 |
| Inhibitor | IC50 Value (mg/mL) |
| Petroleum Ether extract | 10.436 |
| Ethanol extract | 12.644 |
| Constituent | Observation | Inference |
| Alkaloids | Reddish brown colour | Alkaloids present |
| Flavonoids | Yellow precipitate | Flavonoids present |
| Phenols | Blue colour | Phenols present |
| Proteins | No colour change | Proteins absent |
| Tannins | No colour change | Tannins absent |
| Saponins | Presence of Emulsion | Saponins present |
| Glycosides | No colour change | Glycosides present |
| Terpenoids | Yellow colour in lower layer | Terpenoids present |
| Steroids | Red colour in lower layer | Steroids present |
| est | Observation | Inference |
| Alkaloids | Reddish brown colour | Alkaloids absent |
| Flavonoids | Yellow precipitate | Flavonoids absent |
| Phenols | Blue colour | Phenols absent |
| Proteins | No colour change | Proteins absent |
| Tannins | No colour change | Tannins absent |
| Saponins | Presence of Emulsion | Saponins absent |
| Glycosides | No colour change | Glycosides present |
| Terpenoids | Yellow colour in lower layer | Terpenoids absent |
| Steroids | Red colour in lower layer | Steroids absent |
Figure 1.Flow chart showing the preparation of Biuret reagent
Figure 2.BSA standard calibration Curve
Figure 3.Starch standard calibration Curve
Figure 4.Percent Inhibition of alpha amylase by ethanol and petroleum ether extracts at different concentrations
Figure 5.Percent Inhibition of alpha amylase by ethanol and petroleum ether extracts at different concentrations.
Figure 6.Percent Inhibition of alpha amylase by petroleum ether extract at different concentrations.
Figure 7.Percent Inhibition of alpha amylase by ethanol extract at different concentrations.
Figure 8.Michaelis-Menten graph of alpha amylase activity and inhibition by ethanol extract at different concentrations
Figure 9.Lineweaver-Burk plot of type of inhibition of alpha amylase by ethanol extract at IC50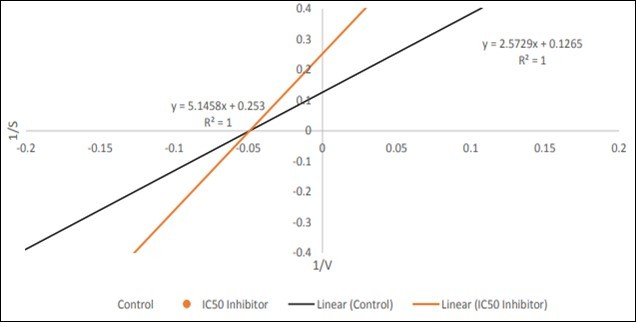
Discussion
Inhibitors of α-amylase delay the breakdown of carbohydrate in the small intestine and diminish the postprandial blood glucose excursion in a person suffering from diabetes. One of the strategies adopted to manage diabetes mellitus involves the inhibition of carbohydrate digesting enzymes such as α-amylase and α-glucosidase in the gastrointestinal glucose absorption thereby lowering postprandial glucose level 18. This research is an attempt to search for alternative drugs from medicinal plants with increased potency and lesser adverse effects than existing drugs.
In this study, the effect of Tridax procumbens leaf extracts on the activity of α-amylase was evaluated. The plant extract showed potent inhibition of α-amylase activity. This result is in agreement with previous reports by 17, which indicated that excessive inhibition of pancreatic α-amylase could result in the abnormal bacterial fermentation of undigested carbohydrates in the colon and therefore mild α-amylase inhibition activity is desirable. Lineweaver-Burk plot also showed that Petroleum ether extract of this plant inhibitsα-amylase non-competitively. This suggests that the extract reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate, thereby preventing the breaking down of oligosaccharides to disaccharides.
This inhibitory activity of the Tridax procumbens leaf extract might be due to the presence of several phytochemicals such as flavonoids, saponins, and tannins that have been determined experimentally in this study and a study by P et al. (2011). Previous studies on alpha amylase inhibitors identified from medicinal herbs state that a number of capable inhibitors belong to flavonoid class that have features of inhibiting -amylase activities. In general, the enzyme inhibitory activity of plant extracts does not just rely on the amount of especial phytochemicals but additionally may depend on the quality of especial phytochemicals. Additionally, researchers also have reported that biological activities of phytochemicals depend on the extent of hydroxylation and conjugation. 10
The Petroleum ether extract of Tridaxprocumbensleaves showed highest level of inhibitory activity on alpha amylase. Upon determination of the IC50 value of the Extracts by the two solvents showed the Petroleum ether extract required a lesser concentration of the extract to produce 50% inhibition of alpha amylase as compared to the ethanolic extract which required higher concentration of the leaf extract. This according to 26 was as a result of the Petroleum ether solvent being able to extract more phytochemicals and other metabolites contained in the leaf samples leading to higher levels of inhibition at lower concentrations. IC50 values for acarbose, a drug currently widely used as medication for the management of diabetes mellitus, was determined to range between 0.26 – 0.29 mg/mL according to research by 2. This concentration produced inhibition of alpha amylase in a range of 66 – 69%. Acarbose also shows maximum inhibition in a range between 70 to 80% at 2.5 mg/mL. Maximum inhibition increases slightly as concentration of acarbose is increased. When analyzed, Tridax procumbensleaf extracts cannot replace acarbose when inhibition needed in management of alpha amylase is smaller, but when high inhibition rates of alpha amylase is needed, Tridax procumbens extracts could be used as they can achieve higher inhibition at higher concentrations than acarbose. This can be applied in clinical conditions and cases where there is high activity of alpha amylase in the patient and hence high inhibition of alpha amylase is needed in order to manage diabetes mellitus.
As determined experimentally the best solvent for the extraction of the Tridax procumbensleaf was petroleum ether as this solvent produced effective extraction of phytochemicals contained in the leaves of Tridax procumbens which led to a higher inhibition of alpha amylase at lower concentrations as compared to the ethanolic extract.
Inhibition was determined to be non-competitive which proved that the extract reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate. Therefore, the enzyme had equal Km values for when there was no extract and when there was, but a reduced Vmax in the presence of extract. In comparison to the positive control acarbose which produced Km values of 11.1 mM addition of extract produced Km value of 20.3 mM. Starch also from standard values has a Km of 0.16 mM. This explains the affinity of the enzyme for the substrate, in that affinity follows as;
Starch > Acarbose > Extract
Conclusion, Limitations and Recommendations
Conclusion
Tridaxprocumbensis a traditional medicinal plant that is popularly used for the management of diabetes in complementary and alternative medicine, and several scientific lines of evidences were reported in favour of the same. The present study established that the leaf extracts from Tridax procumbens do have α-amylase inhibiting activities and glucose lowering activity. The study has also established that extracts could be used in the production of drugs which can help in the management of chronic diabetes mellitus.
Limitation of the study
The study did not cover the quantification of phytochemicals in extracts and the preparation of Acarbose positive control due to limited resources.
Appendix
Appendix 1.Stick model of alpha amylase. 29
Appendix 2.Model of alpha amylase. 1
Appendix 3.Surface model of alpha amylase with Ligand attached. 13
Appendix 4.Tridax procumbens plant and leaves
Appendix 5.Phytochemical analysis
Appendix 6.Alpha amylase inhibitory assay
Appendix 7.Alpha amylase activity and Inhibitory assay by Tridax procumbens extract
Recommendations
Further research is needed to develop anti-diabetic oral drug from this natural source. In Vivo research must be conducted to assay the inhibitory properties of the extract.
Research must be conducted to identify and purify the extracts obtained in order to avoid confounding factors.
References
- 1.Soud Abu, S R, Hamdan I, F U Afifi. (2004) Alpha amylase inhibitory activity of some plant extracts with hypoglycemic activity. , Scientia Pharmaceutica 72(1), 25-33.
- 2.M B Adinortey, Agbeko R, Boison D, Ekloh W, L E Kuatsienu et al. (2019) Phytomedicines Used for Diabetes Mellitus in Ghana: A Systematic Search and Review of Preclinical and Clinical Evidence. Evidence-Based Complementary and Alternative Medicine. 1-23.
- 4.Agrawal S, Mohale D, G S Talele. (2010) Pharmacological activities of Tridax procumbens (Asteraceae). Medicinal Plants -. , International Journal of Phytomedicines and Related Industries 2, 73-78.
- 5.Babayi H, R O Alabi, E D Amali, Baba E. (2018) . Modern Chemistry & Applications Effects of Oral Administration of Aqueous Extract of Tridax procumbens Leaves on Some Haematological Variables in Rats 6(1), 10-4172.
- 6.N M Baldé, Youla A, M D Baldé, Kaké A, M et al. (2006) Herbal medicine and treatment of diabetes in Africa: An example from Guinea. Diabetes and Metabolism.
- 7.Beaupoil-Abadie B, Raffalli M, Cozzone P, Marchis-Mouren G. (1973) Determination of the carbohydrate content of porcine pancreatic amylase. , Biochimica et Biophysica 297(2), 436-440.
- 8.M A Bhutkar, S B Bhise. (2012) In vitro assay of alpha amylase inhibitory activity of some indigenous plants. , International Journal of Chemical Sciences
- 9.Chakrabarti R, Sigh B, P V N, Vanchhawng L, Thirumurugan. (2014) Screening of Nine Herbal Plant for Inhibition α-Amilase Inhibition. , Asian Journal of Pharmaceutical and Clinical Research 7(4), 84-89.
- 10.M F Faizi. (2015) Determination of the effects of some plant extracts on the activity of peroxidase, identification and quantification of constituents contributing to the effects. Revista Brasileira de Geriatria e Gerontologia, III(4) 224-234.
- 11.Funke I, Melzig M F. (2005) Effect of different phenolic compounds on amylase activity: Screening by microplate-reader based kinetic assay. , Pharmazie 60(10), 796-797.
- 12.K B Ishnava. (2018) In - vitro Study on α - amylase Inhibitory Activity of Selected Ethnobotanical Plant Extracts and its Herbal Formulations. , International Journal of Pharmacognosy & Chinese Medicine 2(3), 10-23880.
- 13.M S Islam, M B Aktar, M. (2014) Determination of alpha-amylase activity of Streptomyces spp isolated from Bangladeshi soils. 1(10), 167-170.
- 14.S M Jachak, Gautam R, Selvam C, Madhan H, Srivastava A et al. (2011) Anti- inflammatory, cyclooxygenase inhibitory and antioxidant activities of standardized extracts of Tridax procumbens L. , Fitoterapia 82(2), 173-177.
- 15.Kahanovitz L, P M Sluss, S J Russell. (2017) Type 1 Diabetes - A Clinical Perspective. , Point of Care 16(1), 37-40.
- 16.S E Kahn, M E Cooper, Prato Del, S. (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. , Lancet (London, England) 383(9922), 1068-1083.
- 17.M I Kazeem, J O Adamson, I A Ogunwande. (2013) Modes of inhibition of α -amylase and α -glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Research International. 527570-10.
- 18.M I Kazeem, J O Adamson, I A Ogunwande. (2013) Modes of Inhibition of α - Amylase and α -Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. BioMed Research International. 1-6.
- 19.R P Mahajan. North Maharashtra University (2012) Study of In Vitro Inhibition of α - amylase by Phytochemicals , Aqueous and Ethanol Extracts of Some Medicinal Plants.
- 20.Nair S, Dixit S. (2017) A comparative analysis of in vitro antioxidant potential of crude extracts of Tridax procumbens L. in different solvents and in vitro hypoglycemic potential of its hydro-alcoholic extract. , Pharmaceutical and Biomedical Research 2(3), 47-55.
- 21.Zinjarde P S, Bhargava S, SY, Kumar A R. (2011) Potent $α$-amylase inhibitory activity of Indian Ayurvedic medicinal plants. , BMC Complementary and Alternative Medicine 11(1), 5.
- 22.Zinjarde P S, Bhargava S, SY, Kumar A R. (2011) Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. , BMC Complementary and Alternative Medicine 11(1), 5.
- 23.Manivannan R, Saravanan S. (2016) Isolation, Identification and anti oxidant and anti- inflammatory activities of Tridax procumbens flower extracts. , Journal of Pharmacy Research 10(7), 502-506.
- 24.S.O., O., B.O., O., I.I., I., P.E., O., R.M., C., Aiyegoro O.A. AO - Oyedemi Roger M.; S. O; A. O.-C., … Aiyegoro, O. A. (2017). Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. Scientific World Journal, 2017, 3592491 . .
- 25.Sailaja B, Bharathi K, Prasad K V S R G. (2012) Role of Tridax procumbens Linn . in the management of experimentally induced urinary calculi and oxidative stress in rats. 3, 535-540.
- 26.Saxena M, A H Mir, Sharma M, M Y, Qureshi S et al. (2013) Phytochemical Screening and In-vitro antioxidant activity isolated bioactive compounds from Tridax procumbens linn. , Pakistan Journal of Biological Sciences 16(24), 1971-1977.
- 28.K M Simmons, A W Michels. (2015) Type 1 diabetes: A predictable disease. , World Journal of Diabetes 6(3), 380.
- 29.Sky-Peck H, Thuvasethakul P.Human pancreatic alpha-amylase. II. Effects of pH, substrate and ions on the activity of the enzyme. , Annals of Clinical and Laboratory Science 7(4), 310-317.
- 30.B W Smith, J H Roe. (1949) A photometric method for the determination in blood and urine, with use of the starch iodine color. , The Journal of Biological Chemistry 179, 53-59.
